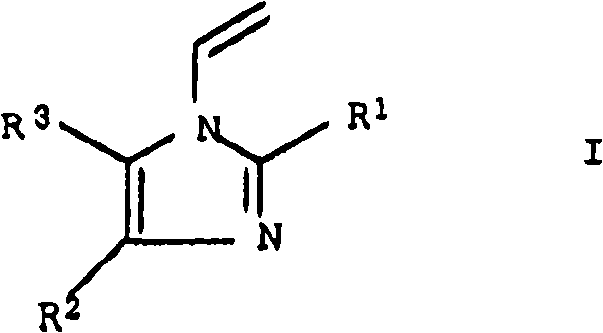
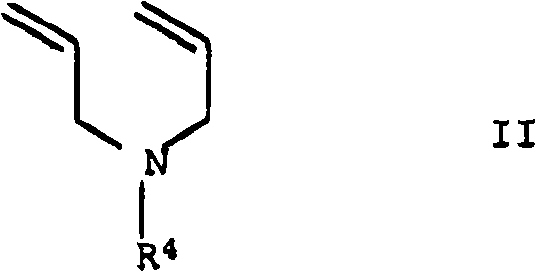
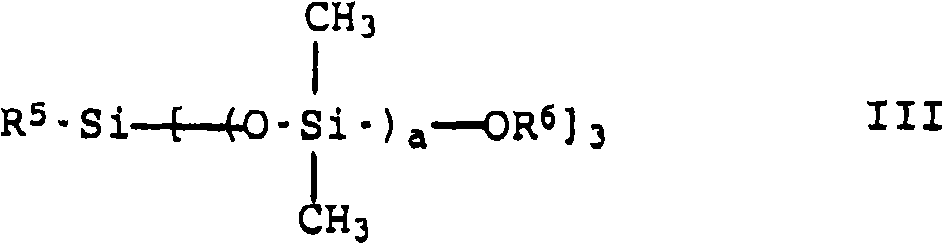Cosmetic or dermatological sunscreens
A technology of protonating agent and quaternizing agent, which is applied in the direction of cosmetic preparations, skin care preparations, cosmetics, etc., and can solve the problems of dispersion and inability to ensure the best application of the skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0140] 400 g of water and 46 g of dimethyldiallylammonium chloride solution (65% strength) were added to a stirrer. 10% of feedstock 1 consisting of 270 g of N-vinylpyrrolidone and 0.6 g of N,N'-divinylethyleneurea was added to this initial charge. The mixture was heated to 60° C. under a stream of nitrogen with stirring, then starting material 1 was metered in over a period of 3 hours, and then 0.9 g of 2,2′-azobis(2-amidinopropane was added over a period of 4 hours to ) dihydrochloride and 100 g of water starting material 2 was metered in. After 3 hours, the mixture was diluted with 700 g of water and stirred for another 1 hour. Then, a solution of 1.5 g of 2,2'-azobis(2-amidinopropane) dihydrochloride in 30 g of water was added and the mixture was stirred at 60° C. for a further 2 hours. This gave a colorless, highly viscous polymer solution with a solids content of 20.9% and a K value of 80.3.
preparation Embodiment 2
[0142] Add 300 g of N-vinylpyrrolidone by 200 g, 77 g of dimethyl diallyl ammonium chloride solution (65% concentration), 1.13 g of N, N'-divinyl ethylene urea and 440 g of water consisted of starting material 1, and the mixture was then heated to 60° C. with stirring and nitrogen flow. The remainder of starting material 1 was metered in over 2 hours, then starting material 2 consisting of 0.75 g of 2,2'-azobis(2-amidinopropane) dihydrochloride and 100 g of water was metered in over a period of 4 hours. When the addition of starting material 1 was complete, the reaction mixture was diluted with 1620 g of water. When the addition of starting material 2 was complete, the mixture was stirred at 60° C. for a further 1 hour, then a solution of 1.25 g of 2,2′-azobis(2-amidinopropane) dihydrochloride in 65 g of water was added, The mixture was stirred for another 1 hour. This gave a colorless, highly viscous polymer solution with a solids content of 10.2% and a K value of 80.
preparation Embodiment 3
[0144] 130 g of water and 48 g of 3-methyl-1-vinylimidazolium chloride were added to a stirrer, and the resulting mixture was heated to 60° C. under a nitrogen stream with stirring. Then, starting material 1 consisting of 192 g of N-vinylpyrrolidone, 0.48 g of N,N'-divinylethylene urea and 450 g of water was metered in over 3 hours, and then 1.44 g of Feedstock 2, consisting of 2,2'-azobis(2-amidinopropane) dihydrochloride and 80 g of water, was metered in. The mixture was then stirred at 60°C for an additional 1 hour. In order to keep the mixture in a stirrable state, it was diluted with a total of 2100 g of water as needed. This gave a colorless, highly viscous polymer solution with a solids content of 8.2% and a K value of 105.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com