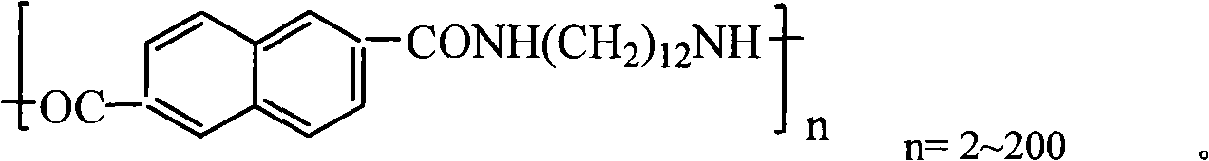Long carbon chain condensed ring semi-aromatic nylon and synthetic process thereof
A synthetic process and semi-aromatic technology, applied in the field of nylon and its synthesis, to achieve the effects of cost reduction, abundant raw material sources, excellent toughness and weather resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Accurately weigh a total of 150g of 2,6-naphthalene dicarboxylic acid and 1,12-dodecadiamine with a molar ratio of 1:1, add it to 1600ml of distilled water, and keep the water temperature at 80°C under conditions of stirring The reaction was carried out at low temperature for 4 hours, and then 1,12-dodecadiamine was added to adjust the pH of the aqueous solution to 7.0. After the pH value stabilized, the reaction was continued at constant temperature for 1.5 hours. The system was cooled at 5°C for 2 hours, and the precipitate was filtered and dried to obtain PA12N nylon salt with a melting point of 259°C.
[0023] The second step: make the PA12N nylon salt obtained in the first step into a 75% aqueous solution, put it in a 1500ml polymerization reaction kettle, replace the air in the kettle with carbon dioxide gas, and keep the carbon dioxide at a pressure of 0.2MPa as a protective gas . The reaction kettle was heated slowly, heated to 226°C within 2.5 hours, a...
Embodiment 2
[0028] Step 1: Accurately weigh a total of 100g of 2,6-naphthalene dicarboxylic acid and 1,12-dodecadiamine with a molar ratio of 1:0.96, add it to 1200ml of distilled water, and keep the water temperature at 65°C under conditions of stirring React at low temperature for 2 hours, add 1,12-dodecadiamine to adjust the pH of the aqueous solution to 7.2, and continue the reaction at a constant temperature for 2 hours after the pH value is stable, and cool the system at 9°C for 3 hours to remove the precipitate The product is filtered and dried to obtain PA12N nylon salt, the melting point of which is 259°C.
[0029] The second step: make the PA12N nylon salt obtained in the first step into a 60% aqueous solution, put it in a 1500ml polymerization reaction kettle, replace the air in the kettle with carbon dioxide gas, and keep the carbon dioxide at a pressure of 0.1MPa as a protective gas . The reaction kettle was heated slowly to 220°C within 2 hours, the pressure was maintained ...
Embodiment 3
[0032] Step 1: Accurately weigh a total of 200g of 2,6-naphthalene dicarboxylic acid and 1,12-dodecandiamine with a molar ratio of 1:1.02, add it to 2000ml of distilled water, and keep the water temperature at 95°C with stirring React at low temperature for 5 hours, add 1,12-dodecadiamine to adjust the pH of the aqueous solution to 7.5, and continue the reaction at a constant temperature for 3 hours after the pH value is stable, and then cool the system at 0°C for 2 hours. The precipitate is filtered and dried to obtain PA12N nylon salt with a melting point of 259°C.
[0033] The second step: make the PA12N nylon salt obtained in the first step into an 85% aqueous solution, put it in a 1500ml polymerization reaction kettle, replace the air in the kettle with carbon dioxide gas, and keep 0.4MPa carbon dioxide as a protective gas. The reaction kettle was heated slowly, heated to 250°C within 3 hours, the pressure was kept at 2.0MPa, and maintained at this state for 2 hours, then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com