Ionic membrane caustic soda light salt brine fine purification technique
A technology of ionic membrane caustic soda and light brine, applied in the direction of alkali metal chloride, etc., can solve the problems of increasing salt mud discharge, Na2SO3 waste, NaClO waste, etc., and achieve the reduction of equipment investment, reduction of addition amount, and reduction of external discharge Effect
Active Publication Date: 2008-10-29
昊华宇航化工有限责任公司
View PDF0 Cites 16 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
1. It is necessary to add reducing substance Na2SO3 to the dechlorination system; it not only causes waste of Na2SO3, but also needs to increase Na2SO3 preparation equipment and pipelines, and the process is complicated
2. The added Na2SO3 increases the impurity content of SO4 2- in the brine system. The existence of SO4 2- will not only affect the electrolysis efficiency and the service life of the membrane, but also need to add BaCl2 to remove it, which increases the production cost and the discharge of salt mud
3. In the brine refining process, NaClO needs to be added to remove organic matter and bacteria and algae impurities, which not only causes waste of NaClO, but also requires additional NaClO preparation equipment and pipelines, and the process is complicated
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
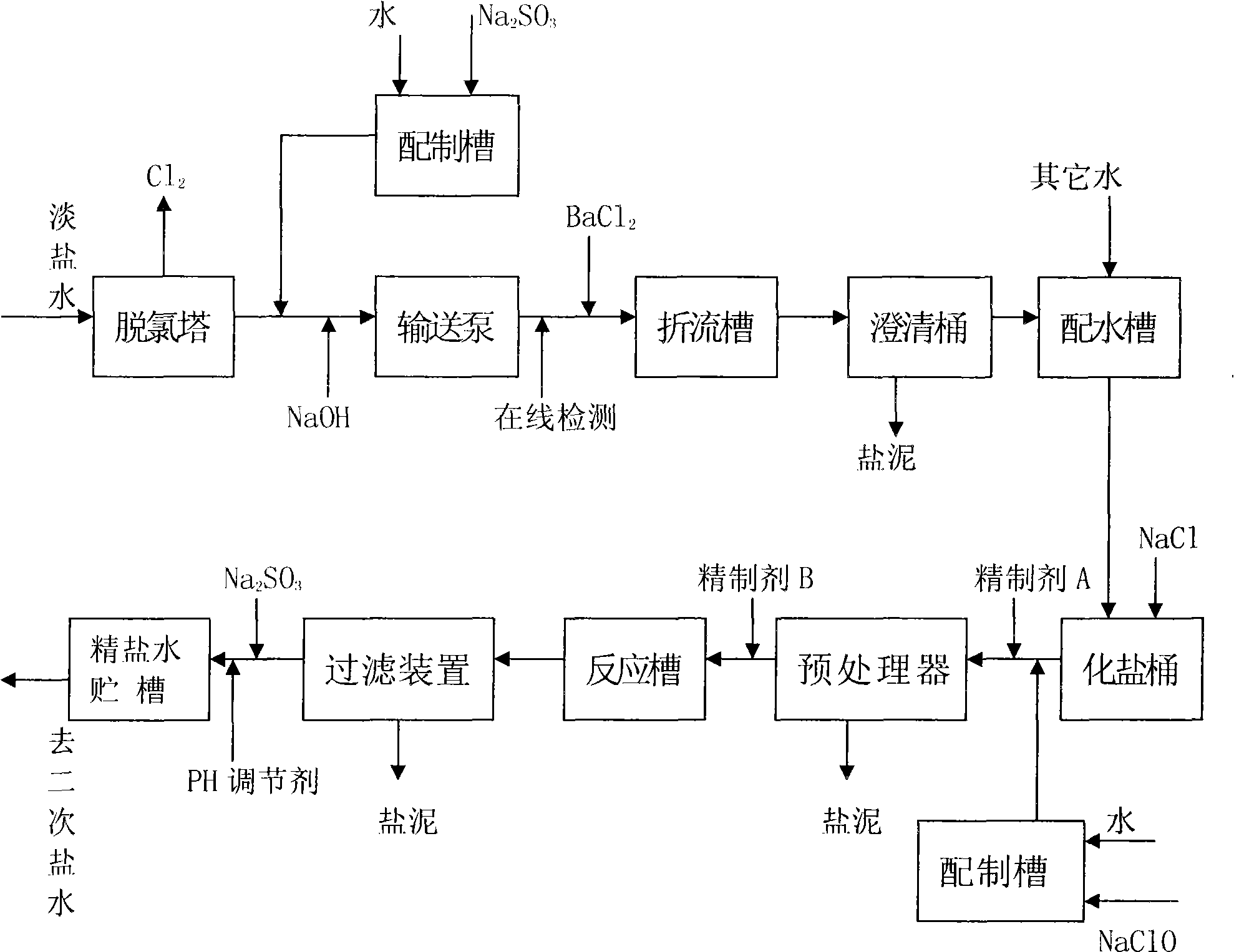
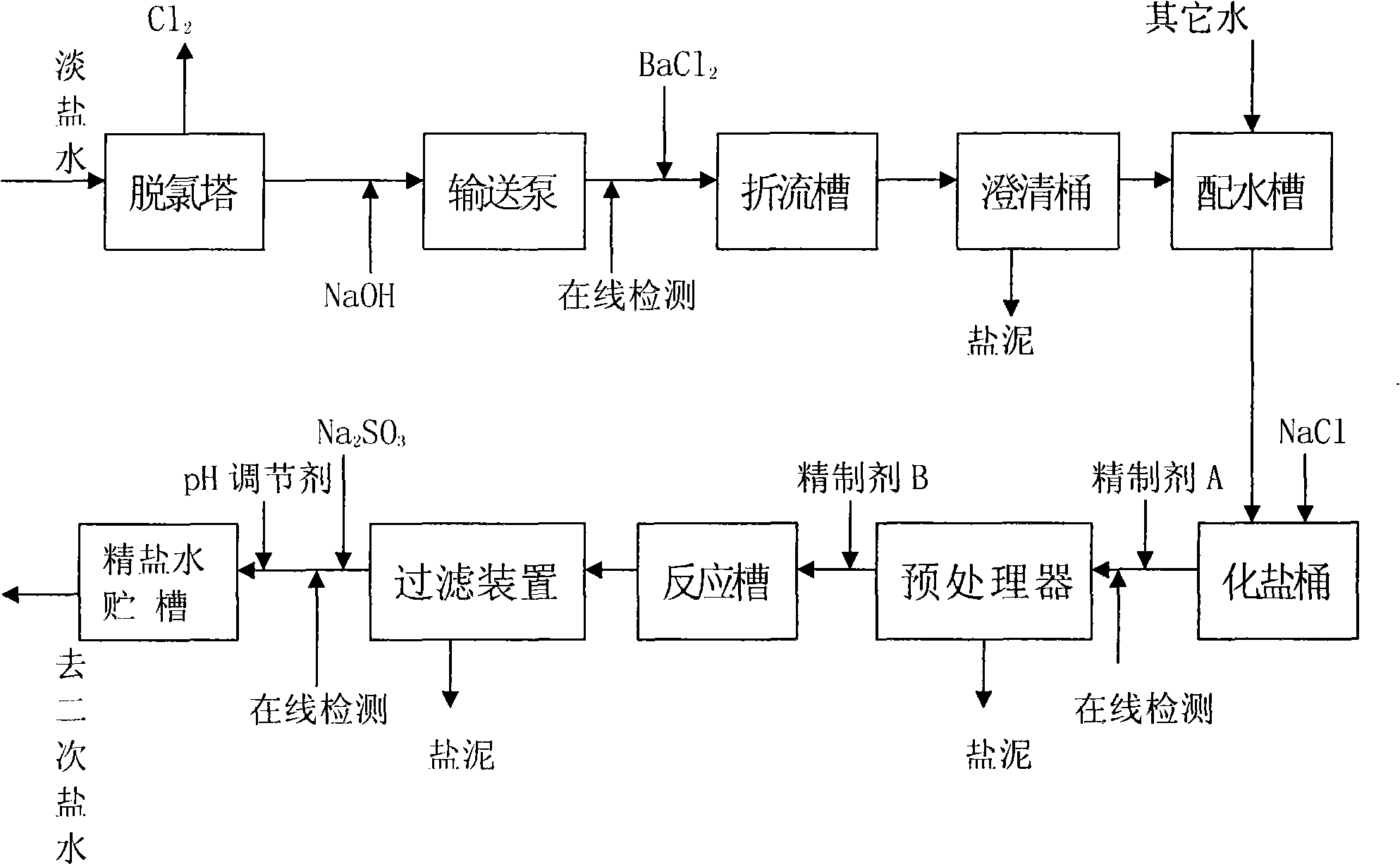
The invention relates to a refining process for fresh brine of ion film caustic soda, in particular to dechlorination of the fresh brine and desorption of organics in the fresh brine; wherein, the fresh brine from an ion-exchange membrane electrolyzer is dechlorinated by a dechlorination tower to a range that the process allows, sent to a first brine process, added barium salt to remove sulfate radical brought in the system by material salt, and then is sent to a salt dissolving tank after water distribution; crude brine from the salt dissolving tank is added a refining agent for purification, Na2SO3 for dechlorination and a PH regulator in sequence, and then is sent to a second brine process after processing. The process is simple, reduces the process of adding Na2SO3 in a dechlorination system and that of adding NaClO in a brine refining system, reduces the variety and quantity of additives, decreases equipment investment and correspondingly saves power consumption of the part as well as alleviates the environmental pollution.
Description
Ion-exchange membrane caustic soda dilute brine refining process (1) Technical field The invention relates to an ion-exchange membrane caustic soda dilute brine refining process, in particular to the dechlorination of dilute brine and the removal of organic matter therein. (2) Background technology In the production of ion-exchange membrane caustic soda, the dilute brine from the ion-exchange membrane electrolyzer must be returned to the smelting system for smelting. However, since the dilute brine contains a large amount of free chlorine, in order to reduce the corrosion of the equipment and pipelines of the brine system, It needs to go through the dechlorination tower for dechlorination, but after dechlorination, it still contains about 10-100mg / L of free chlorine (mainly in the form of ClO-), and then add the reducing substance Na2SO3 and ClO- to react to completely remove the free chlorine. ClO-+SO3 2-→SO4 2-+Cl- After thorough dechlorination, the dilute brine is sent to a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C01D3/04
Inventor 刘志强赵述彬郭金星王志勇
Owner 昊华宇航化工有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com