Preparation of clindamycinum phosphoester
A technology for clindamycin phosphate and lincomycin hydrochloride, which is applied in the field of preparation of clindamycin phosphate, can solve the problems of high environmental protection treatment pressure, high production cost, and high content of table clindamycin in finished products. Environmental pressure, reducing impurities and production costs in finished products, and reducing the effect of epicrine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
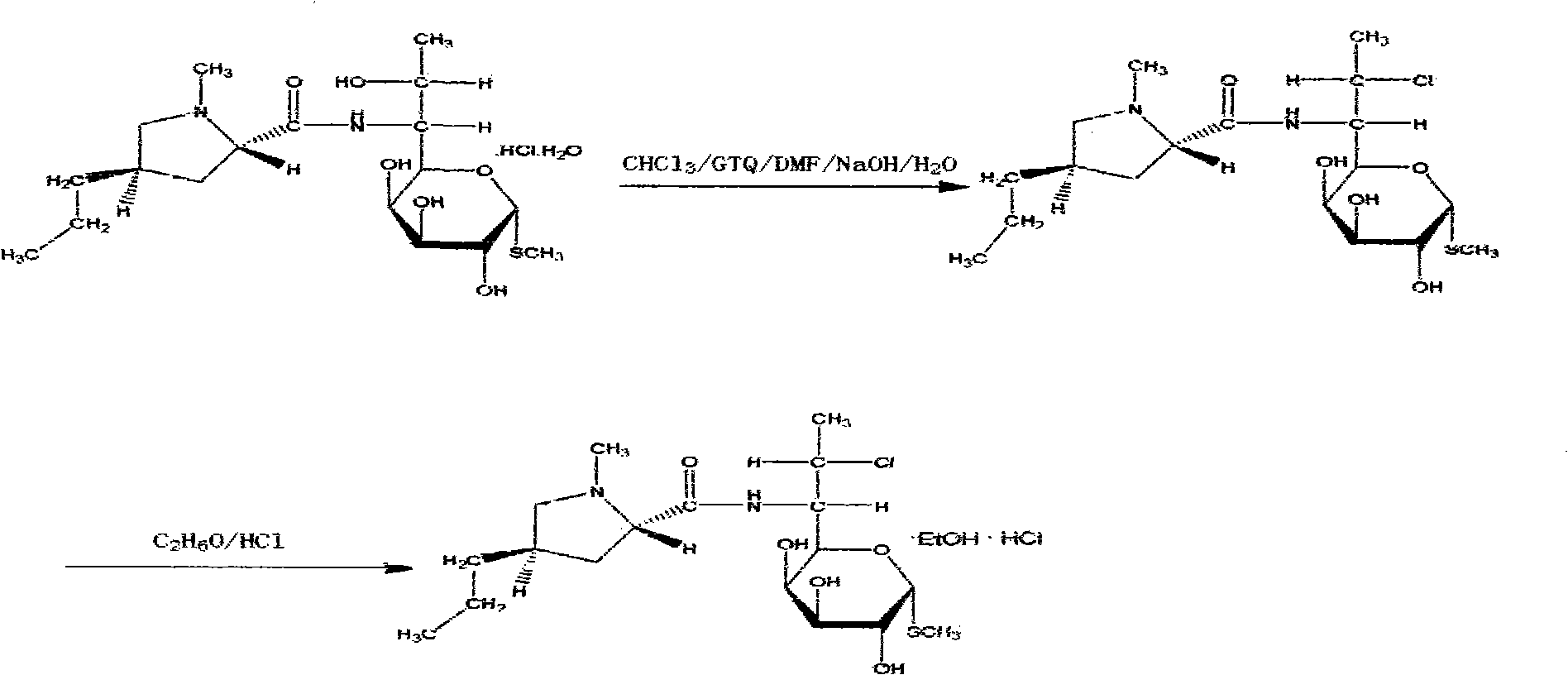
[0030] (1) Chlorination reaction: Take 160 billion lincomycin hydrochloride and 1000 L of chloroform, 227.2 kg of solid light, 1 kg of antioxidant AT-10, and 240 L of DMF at 50-80 ° C for 15 hours. Then lower the temperature to 0°C.
[0031] (2) Alcoholization reaction: add drinking water 800L and mass concentration to the product of step (1) and be that 30% lye (sodium hydroxide aqueous solution) 400L is hydrolyzed at 55 ℃, layering, divide with the chloroform of 272L The organic layer was extracted twice, washed with water, concentrated at 75°C to obtain 1000L of chloroform, and then 160L of absolute ethanol was added for crystallization to obtain alcoholate. The alcoholate yield is 93%.
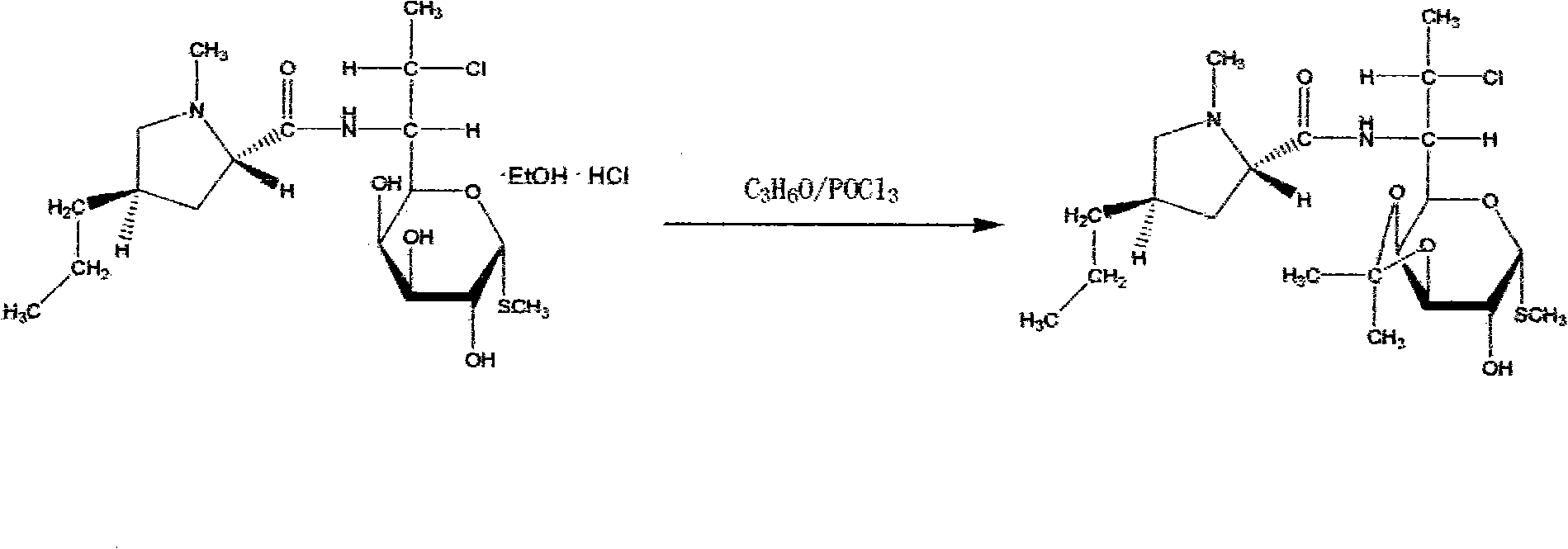
[0032] (3) Ketonization reaction: take 80Kg of the alcoholate obtained in step (2) and react with 320L of acetone and 29L of phosphorus oxychloride at -10°C for 6 hours;
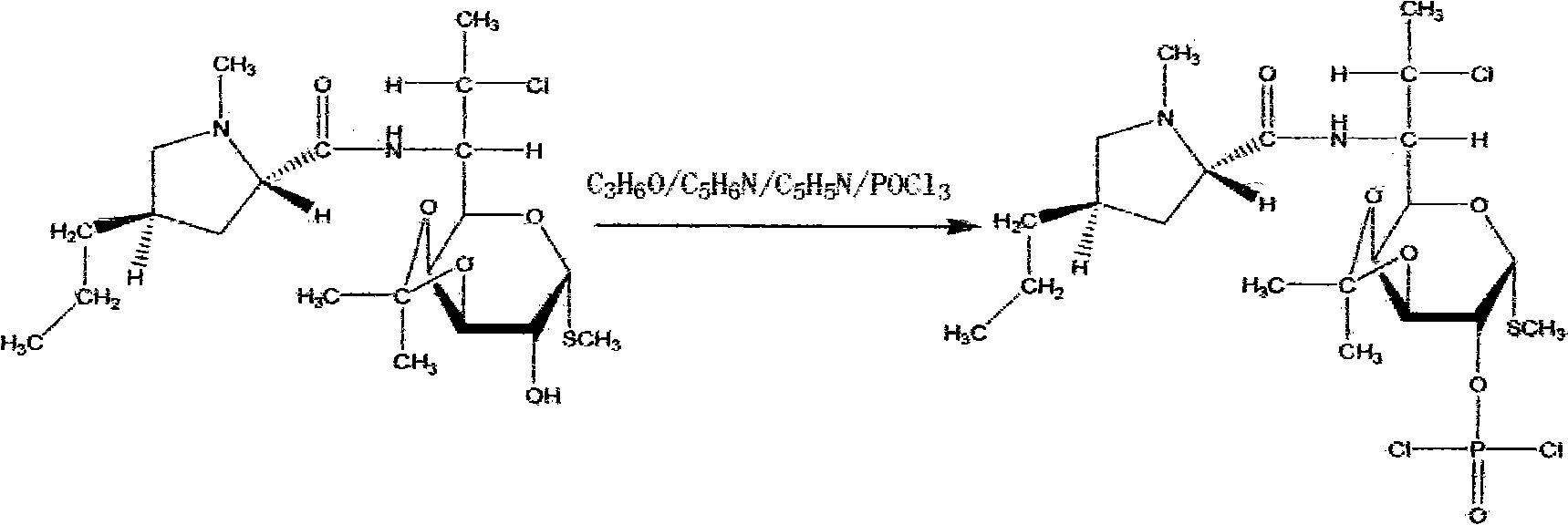
[0033] (4) Esterification: react with 56L of phosphorus oxychloride, 80L of inhaled acetone, 60L of pyridine, and 70...
Embodiment 2
[0036] (1) Chlorination reaction: Take lincomycin hydrochloride 80 billion and chloroform 500L, solid light 113.6kg, antioxidant AT-100.5kg, DMF 120L at 50-80 ℃, heat preservation reaction for 25 hours. Then lower the temperature to 0°C.
[0037] (2) Alcoholization reaction: 400L of drinking water and 200L of 30% lye (sodium hydroxide aqueous solution) are hydrolyzed at 20°C in the solution obtained in step (1), and the layers are separated, and the organic layer is extracted twice with 136L of chloroform , washed with water, concentrated at 50°C to get 500L of chloroform, and then added 80L of absolute ethanol for crystallization to obtain alcoholate. Its alcoholate yield is 85%.
[0038] (3) Ketonization reaction: take 40Kg of the alcoholate obtained in step (2) and react with 160L of acetone and 14.5L of phosphorus oxychloride at 20°C for 7 hours;
[0039] (4) Esterification reaction: react with 28 L of phosphorus oxychloride, 40 L of acetone, 30 L of pyridine, and 35 L o...
Embodiment 3
[0042] (1) Chlorination reaction: Take lincomycin hydrochloride 240 billion and chloroform 1500L, solid light 340.8kg, antioxidant AT-10 1.5kg, DMF 360L at 50 ~ 80 ℃, heat preservation reaction for 40 hours. Then lower the temperature to 0°C.
[0043] (2) Alcoholation reaction: 1200L of drinking water and 600L of 30% sodium hydroxide solution are hydrolyzed at 40°C in the solution gained in step (1), and the layers are separated, and the organic layer is extracted twice with chloroform of 408L, washed with water, and Concentrate 1500L of chloroform at 65°C, then add 240L of absolute ethanol to crystallize to obtain alcoholate. Its alcoholate yield is 87%.
[0044] (3) Ketonization reaction: take 120Kg of the alcoholate obtained in step (2) and react with 480L of acetone and 43.5L of phosphorus oxychloride at 0°C for 8 hours;
[0045] (4) Esterification: react with 84 L of phosphorus oxychloride, 120 L of acetone, 90 L of pyridine, and 105 L of triethylamine at 0° C. for 12 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com