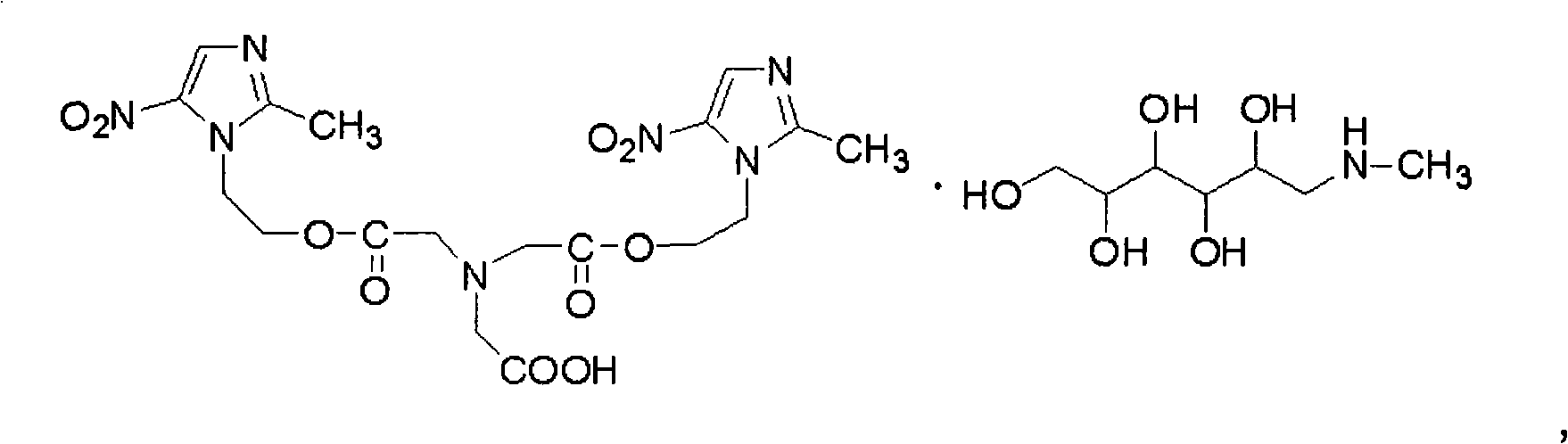
Freeze-dried injection of Meglumine Glycididazole and preparation thereof
A technology of glycidazole meglumine and glycidazole meglumine is applied in the field of freeze-dried powder injection of glycidazole meglumine and its preparation, and can solve the problems of inconvenient storage, poor stability and the like , to achieve the effect of convenient transportation, ensuring stability and improvement, and avoiding adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Glycindiazole Meglumine 310g
[0065] Lactose 50g
[0066] Mannitol 40g
[0067] Sodium dihydrogen phosphate amount
[0068] Add water for injection to 2800ml
[0069] Take the prescription amount of meglumine, lactose, and mannitol and add 2500ml of water for injection to dissolve, add 1M sodium dihydrogen phosphate aqueous solution to adjust the pH to 7.8, add water for injection to 2800ml, and filter to sterilize with a microporous membrane. Carry out aseptic filling into 1000 vials under the condition of grade 100. After checking the content, add rubber stoppers, leave holes, and send the glass vials into a sterilized freeze-drying box for freeze-drying. Pre-freeze for 5 hours. Drop to -35, the first sublimation takes 8 hours, and the temperature rises to -5°C; the second sublimation takes 7 hours, the temperature rises to 25°C, take out the vacuum cap or nitrogen-filled cap, and label the aluminum cap. The finished product, each bottle of lyophilized powder for...
Embodiment 2
[0071] Glycindiazole Meglumine 150g
[0073] Glucose 40g
[0074] Edetate Calcium Sodium 0.15g
[0075] Hydrochloric acid (0.1M) appropriate amount
[0076] Add water for injection to 2000ml
[0077] Take the prescription amount of meglumine glycididazole acid, sodium chloride, glucose, add 2800ml of water for injection to dissolve, then add calcium and sodium edetate to dissolve, adjust the pH to 8-9 with 0.1M hydrochloric acid, add water for injection to 2000ml, and use Stir 0.3% (w / v) needles with activated carbon, stir at 45°C for 30 minutes, decarbonize with a sterilized 0.6 μm filter membrane, filter and sterilize with a 0.22 μm filter membrane, measure and adjust the pH to 8-9, The filtrate is filled in 1000 vials, sent to a freeze dryer for freeze-drying, capped or filled with nitrogen, then capped and taken out, and then labeled. The freeze-drying curve is as follows:
[0078] process
[0079] Each bottle of the above-prep...
Embodiment 3
[0081] Glycindiazole Meglumine 600g
[0082] Sorbitol 100g
[0083] Sodium sulfite 12g
[0084] Phosphoric acid (solution) appropriate amount
[0085] Add water for injection to 4000ml
[0086] The preparation method is the same as in Example 1 except that the auxiliary materials are added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com