N-benzoyl rhodamine B hydrazine, preparation and use thereof
A technology for the reaction of benzoyl and benzoyl chloride, which is applied in the fields of fluorescence/phosphorescence, organic chemistry, color/spectral characteristics measurement, etc., can solve the problems of application limitations, susceptibility to interference, poor selectivity, etc., and improve detection sensitivity and selectivity performance, broaden the scope of application, and high detection sensitivity
Inactive Publication Date: 2010-09-29
INST OF CHEM CHINESE ACAD OF SCI
View PDF4 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The disadvantages of these methods are mainly poor selectivity, easy to be disturbed, and their application has been greatly restricted.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Login to View More
Abstract
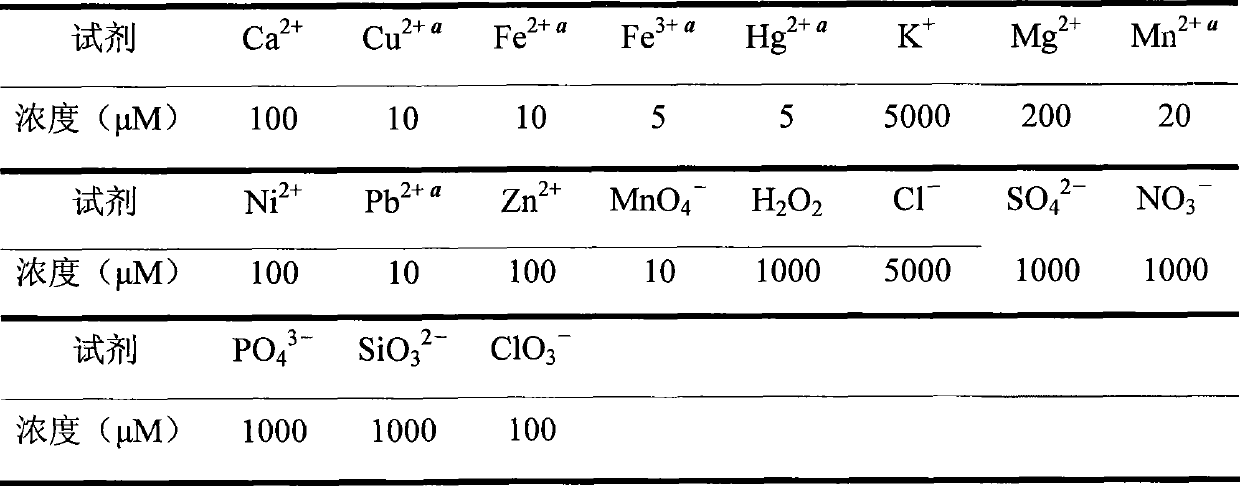
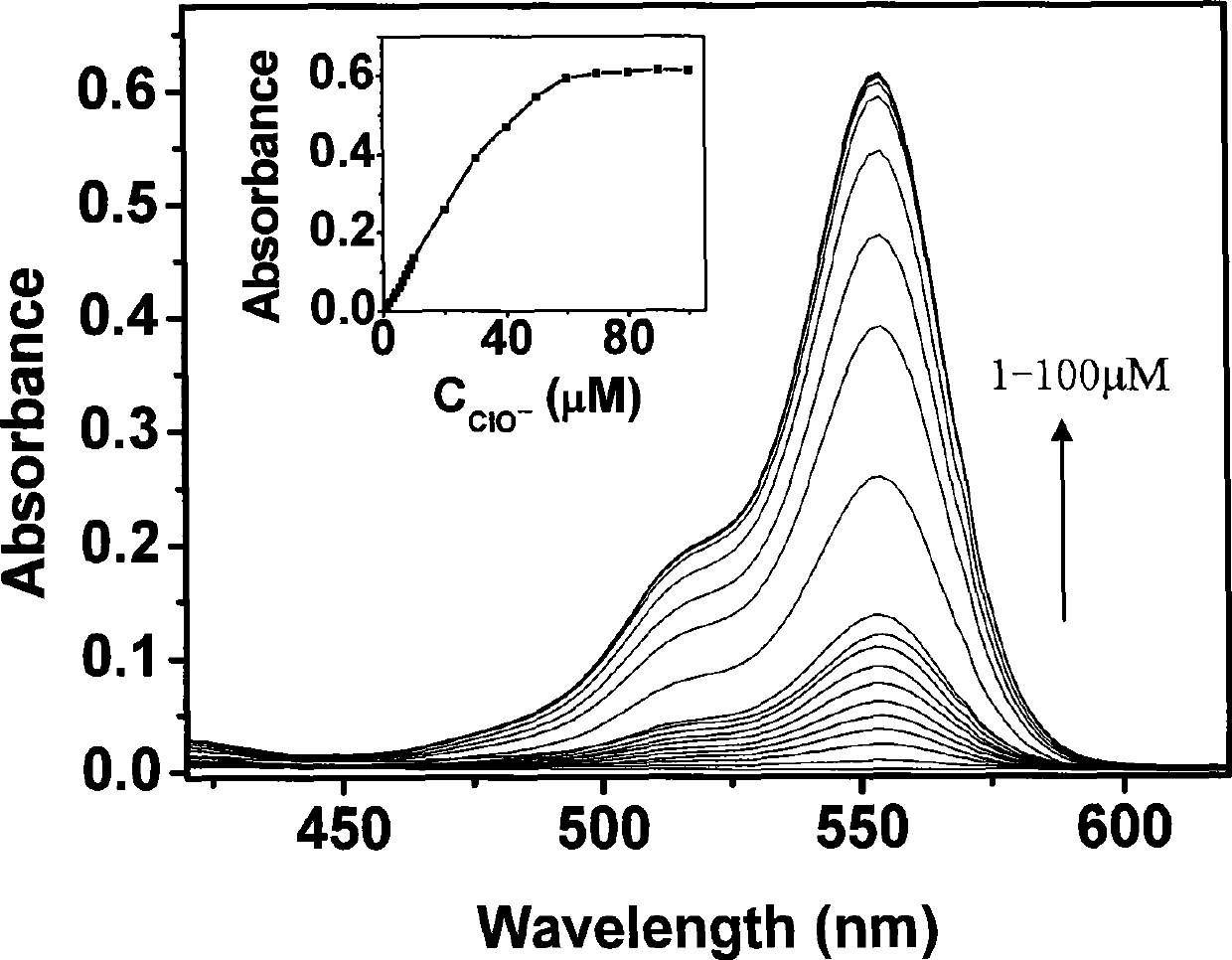
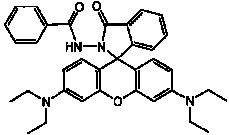
The invention discloses N-benzoyl rhodamine B hydrazide, a method for making the same and an application of the same. The structure of the N-benzoyl rhodamine B hydrazine is shown in formula I. Due to having great molar absorptivity and high fluorescence quantum yield, xanthene dye is an excellent matrix of an optical probe. The xanthene dye derivative-N-benzoyl rhodamine B hydrazide can be selectively reacted with hypochlorite to carry out chromogenic and fluorescence opening reaction; therefore, a system with excellent optical performance can be generated by colorless matter; moreover, detection can be carried out through both a light absorption method and a fluorescence method. In addition, when the N-benzoyl rhodamine B hydrazide is used to detect hypochlorite, high detection sensitivity can be obtained and the detection can be completed just through adopting a small amount of samples, thereby broadening the application range of the method.
Description
technical field The invention relates to N-benzoyl rhodamine B hydrazine and a preparation method thereof, as well as the application of N-benzoyl rhodamine B hydrazine in detecting hypochlorite ions. Background technique The current methods for detecting hypochlorite mainly include sodium thiosulfate titration (HG / T2498-93), o-toluidine colorimetry (GB5750-85) and tetramethylbenzidine (TMB) colorimetry, etc. The disadvantages of these methods are mainly poor selectivity, easy to be interfered, and their application has been greatly limited. As an important fluorescent material, xanthene dyes are widely used in the design and synthesis of various analytical reagents due to their excellent light absorption and fluorescence properties (J. Am. Chem. Soc. 1997, 119: 7386-7387). Taking xanthene dyes as the matrix and modifying them with special reactive groups can combine the high sensitivity of xanthene dyes with the high selectivity of special groups to obtain excellent analy...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D491/10G01N21/64G01N21/31
Inventor 马会民陈新启
Owner INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com