Senile dementia recombinant protein vaccine and preparation method thereof
A recombinant protein and protein vaccine technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problem of unfavorable B cell antigen epitope exposure, difficult chemical modification of synthetic peptides, and impact on antigen space. structure and other problems, to avoid high difficulty and high cost, enhance immunogenicity, and avoid the effect of space rigid structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] 1 recombinant plasmid pQE-4×Aβ 15 preparation of
[0023] 1.1 Design of tetravalent B cell antigen epitope fragment 4×Aβ inserted into pQE-30 15 The sequence is: Aβ 1-15 +GG+Aβ 1-15 +GSSG+Aβ 1-15 +GG+Aβ 1-15
[0024] 1.2 Recombinant plasmid pQE-4×Aβ 15 Construct
[0025] 1.2.1 Aβ 1-15 +GG+Aβ 1-15 +GSSG(Sac I)(2×Aβ 15 -1, the first bivalent Aβ 1-15 gene) fragment primer design
[0026] P1 Primer: 5'-CG GAT GCA GAA TTC CGA CAT GAT TCAGGA TAT GAA GTT CAT CAT CAA GAT GCA GAA TTC CGACAT GAT TCA GGA TAT GAA GTT CAT CAT CAA GG G -3'. The bold italics are the glycine-linked peptides, and the front and back of the glycine-linked peptides are respectively Aβ 15 Base sequences, the bolded BamHI and Sac I restriction sites, the underlined bases for primer pairing.
[0027] P2 Primer: 5'- C CC TTG ATG ATG AAC -3'; Bold is the Sac I restriction site, and the underline is the base paired by the primer;
[0028] 1.2.2GSSG(Sac I)+Aβ 1-15 +GG+Aβ 1-15 (2×Aβ ...
example example 2
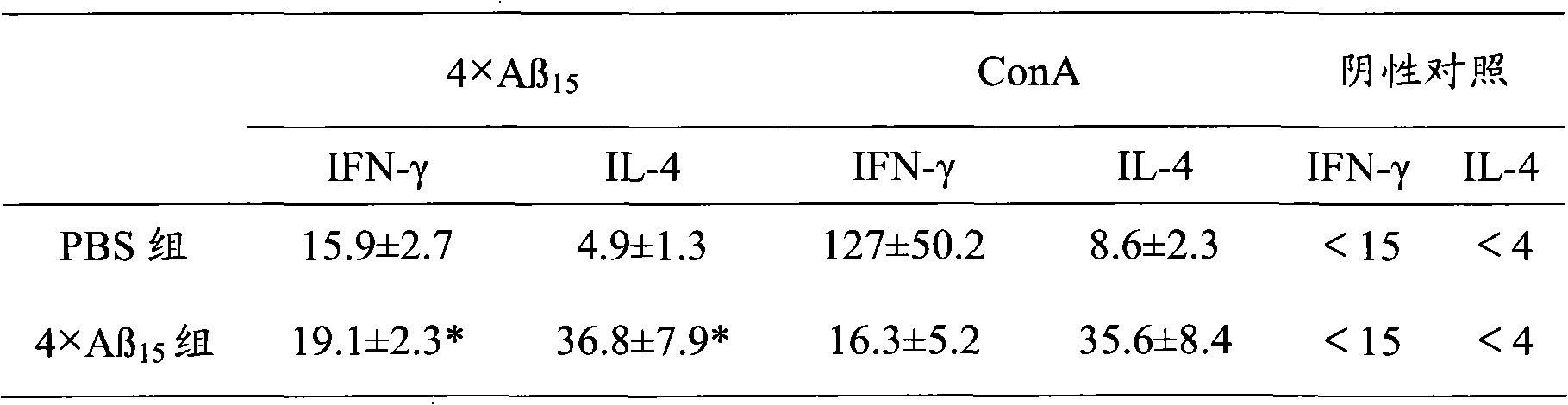
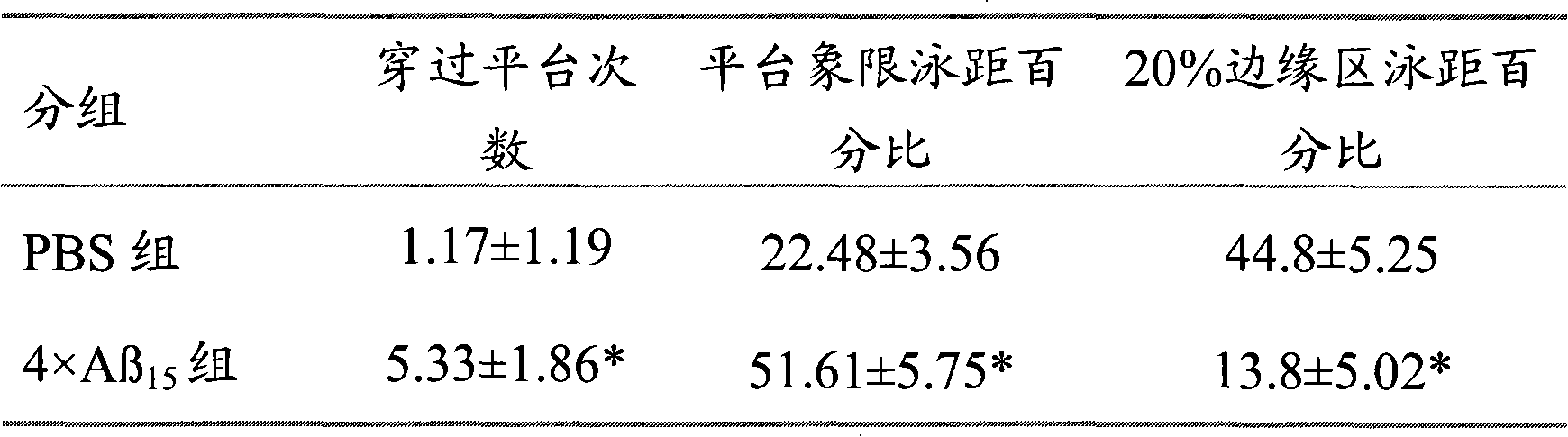
[0044] Example 2 Recombinant 4×Aβ 15 Observation of immunology, pathology and behavior of Tg2576 transgenic mice after protein vaccination.
[0045] 1 Materials and methods
[0046] 1.1 Experimental animals Twelve 12-month-old Tg2576 transgenic mice (Tg2576 transgenic mice are products of Taconic, USA, and were successfully bred in our laboratory). After feeding to 12 months old, they were randomly divided into 2 groups: 4×Aβ 15 group and PBS control group, with 6 rats in each group.
[0047] 1.2 Immunization with recombinant 4×Aβ 15 The protein was mixed with an equal amount of adjuvant MF59 (5ml squalene, 0.5ml Tween80, 0.5ml span85, 94ml PBS to make a 100% mixture, mixed and emulsified for 15min) and fully emulsified to prepare recombinant 4×Aβ 15 The protein vaccine was inoculated with 100 μg / time subcutaneous multi-point injection in six 12-month-old Tg2576 mice, each injection was 100 μL. After the first inoculation, it was boosted two weeks later, and then inoculate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com