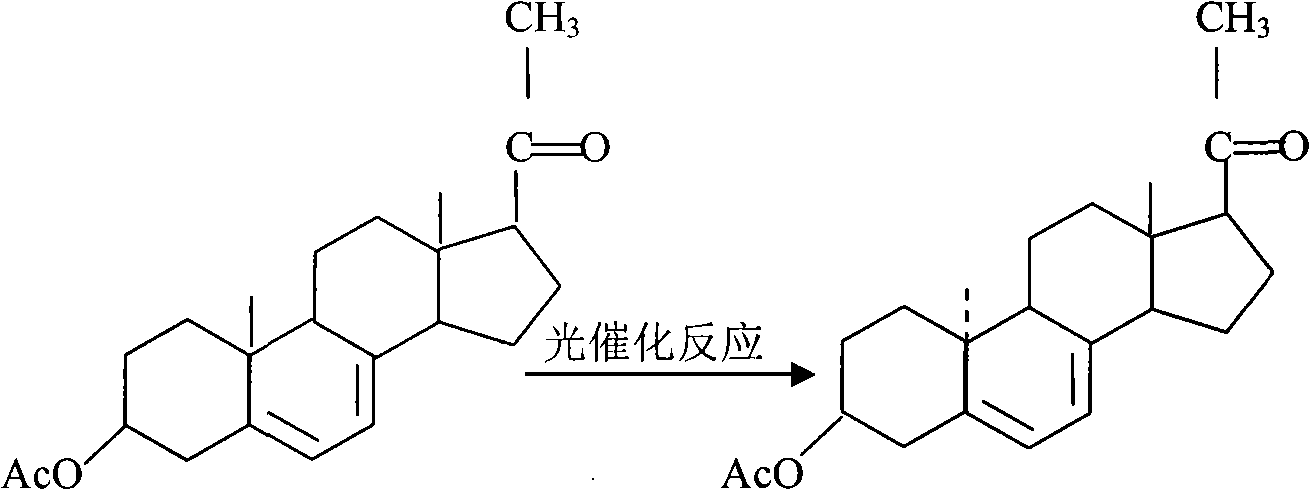
Process for synthesizing 3-acetoxy-9 beta, 10 alpha-pregna-5, 7-diene-20-ketone
A technology of acetoxy and synthetic methods, applied in the field of chemical drug intermediates of dehydroprogesterone, can solve problems such as large safety hazards, difficult industrial production, low light conversion rate, etc., achieve reduced emissions, mild reaction conditions, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 10kg of 3-acetoxy-pregna-5,7-dien-20-one in 1000L ethanol, flow through a quartz reactor, select a light source with a wavelength of 275-295nm, and irradiate with ultraviolet light for 5 hours to obtain a suitable After the product is produced, its solution is added to the photosensitizer anthracene, and the amount of anthracene in the solution is controlled at 0.01g / L, and flows through 10m 2 Quartz three-dimensional coil with a diameter of 1cm is irradiated in an irradiator with an external fluorescent lamp with a wavelength of 300-313nm, the flow rate is controlled at 1000mL / min, and the irradiation temperature is 0-20°C. The irradiated solution flows into the concentration tank and is concentrated to dryness under reduced pressure. The residue in the tank is dissolved with 100L toluene, the insoluble matter (unreacted raw materials) is filtered off, the filtrate is concentrated to dryness under reduced pressure, and 300L acetone and an appropriate amount of ...
Embodiment 2
[0027] Dissolve 10kg of 3-acetoxy-pregna-5,7-dien-20-one in 2000L ethanol, flow through a quartz reactor, select a light source with a wavelength of 275-295nm, and irradiate with ultraviolet light for 4 hours to obtain a suitable After the product is produced, its solution is added to the photosensitizer anthracene. The amount of anthracene in the solution is controlled at 0.5g / L, and the flow through 10m 2 Quartz three-dimensional coil with a diameter of 1cm is irradiated in an irradiator with an external fluorescent lamp with a wavelength of 300-313nm, the flow rate is controlled at 1500mL / min, and the irradiation temperature is 0-20°C. The irradiated solution flows into the concentration tank and is concentrated to dryness under reduced pressure. The residue in the tank is dissolved with toluene, and the insoluble matter (unreacted raw materials) is filtered off. minutes, filtered, concentrated, crystallized by cooling, centrifuged, and dried to obtain 3.2 kg of 3-acetoxy-9...
Embodiment 3
[0029] With reference to the process steps of Example 1, the difference is that 10 kg of the raw material 3-acetoxy-pregna-5,7-dien-20-one is dissolved in 1500L ethanol, and the amount of the solution added to the photosensitizer anthracene solution is controlled at 0.2 g / L, control flow 1200mL / min, 3.5kg yield 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com