Active carbon loading ruthenium ammonia synthesis catalyst and preparation method thereof
A technology of activated carbon and catalyst, which is applied in the field of new ammonia synthesis catalyst and its preparation, can solve the problems of slow reaction speed, incomplete salt, loss of ruthenium, etc., and achieve the effect of improving stability, reducing loss and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
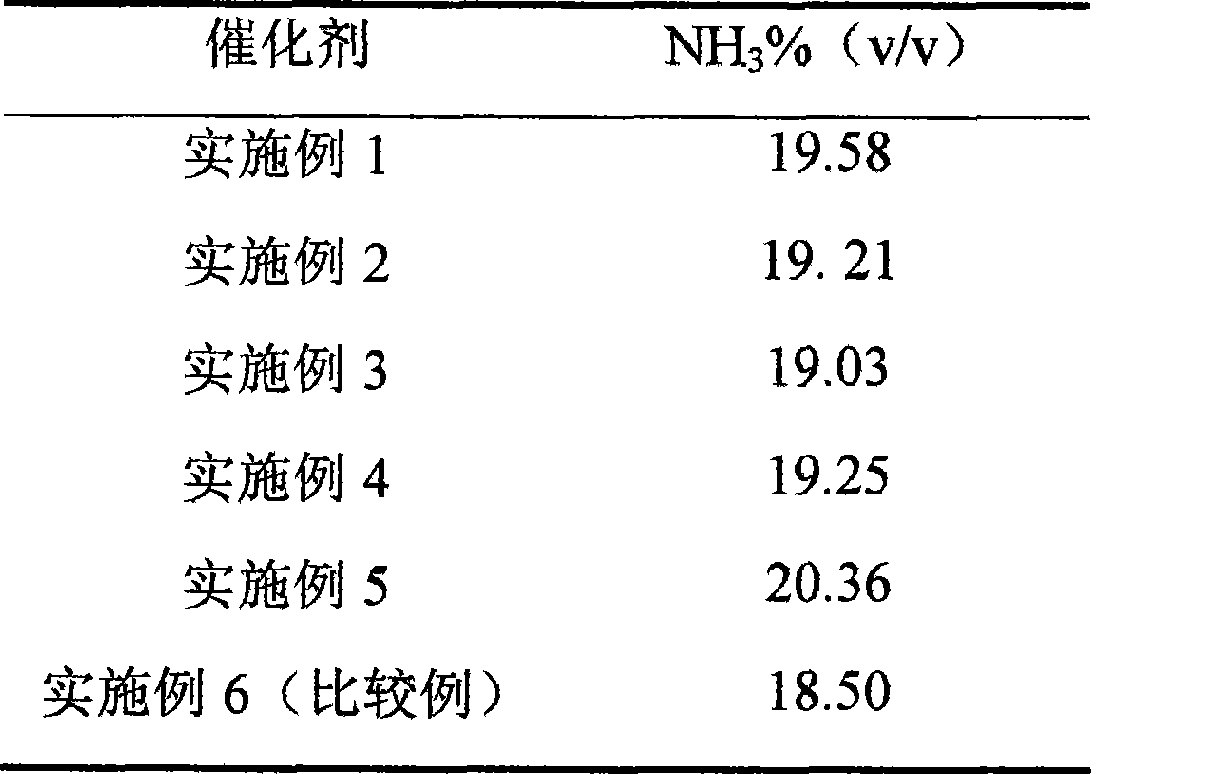
Examples
Embodiment 1
[0043] Take 500 grams of BET specific surface area is 1050m 2 / g of coconut shell charcoal, placed in a washing tower and washed with flowing pure water until the pH value = 7; the activated carbon taken out was dried in a drying oven at 120°C for 4 hours, and then placed in a high-temperature oven under the protection of an inert gas Treat at 1800-1900°C for 2 hours, after the temperature is lowered to room temperature, take it out and place it in a hole-enlarging furnace, and feed it with oxygen, CO 2 The mixed gas composed of nitrogen, nitrogen and water vapor is treated at 380-460°C for 16 hours and then cooled to room temperature for use.
[0044] Get the ruthenium trichloride aqueous solution 200ml that ruthenium concentration is 2.5% (w / w), add concentration and be 30% H 2 o 2 25ml of the solution, stir well at room temperature ~ 95°C and let it stand still, then the Ru in the solution +3 converted to Ru +4 .
[0045] Get 100g of the above-mentioned activated carbo...
Embodiment 2
[0047] The process of Example 1 was repeated except that the impregnation order of each material was different. That is, the treated activated carbon support was first impregnated with RuCl 4 solution and precipitated with KOH solution, washed with deionized water and dried, impregnated with 100ml of oxalic acid solution with a concentration of 12%, dried at 60-120°C with an infrared lamp, impregnated with Ba(NO 3 ) 2 The aqueous solution is based on the weight of the carbon carrier, and the Ba content of the carrier is controlled to be about 4wt%. It is dried at 60-120°C with an infrared lamp or an oven, and the obtained sample is impregnated with a KOH solution at room temperature. The amount of KOH is the same as in Example 1. The sample Drying at 60-120° C. with an infrared lamp can obtain the activated carbon-supported ruthenium-based ammonia synthesis catalyst.
Embodiment 3
[0049] The procedure of Example 2 was repeated, except that oxalic acid was not used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com