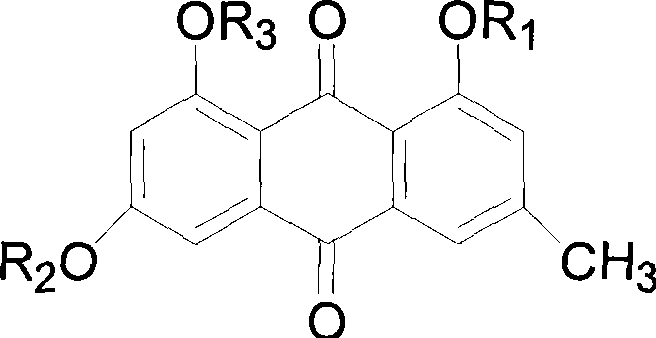
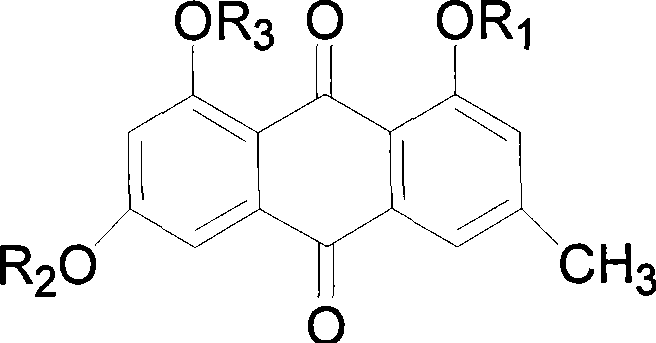
Emodin derivates and application thereof in anti-cancer medicine preparation
A derivative, emodin technology, applied in the field of emodin derivatives and its application in the preparation of anticancer drugs, can solve the problem of insufficient anticancer activity of emodin, and achieve strong cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
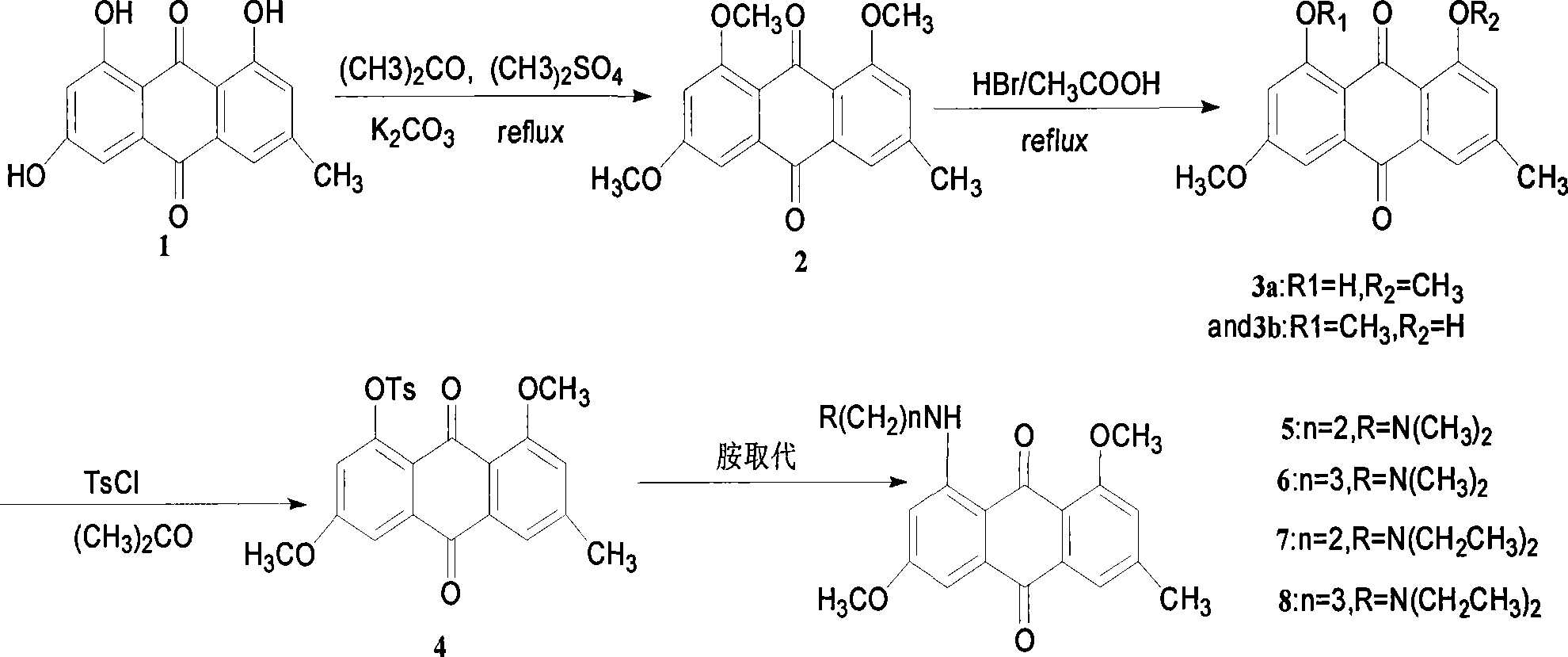
[0018] Separation, extraction and purification of emodin (1).
[0019] Weigh 1Kg tiger stick, crush it through a 20-mesh sieve, use 95% industrial alcohol, reflux and extract twice on a water bath, combine the filtrate, concentrate under reduced pressure to 500ml, add 100-500ml of 3M sulfuric acid, reflux for 0.5-1h, then add 1500ml of water , standing overnight, and dried by suction to obtain 180g of pink powder. Put the obtained red powder into a Soxhlet extractor, and extract it with ether until the ether is nearly colorless in the Soxhlet extractor, first use 5% (w / w) NaHCO 3 solution (6×100ml), the ether layer was extracted, and the aqueous phase was discarded. 5% (w / w) Na 2 CO 3 Solution (8 × 100ml), extract the ether layer, 2 × 100ml of the extract before combining with 5Mol. Hydrochloric acid to adjust pH<4, filter, dry, and crystallize through acetone to obtain 2.75g of orange crystals of compound emodin (1), the product was obtained by Confirmed by IR, NMR, Ms an...
Embodiment 2
[0022] Preparation of Intermediate (2).
[0023] Weigh 2.1g (7.8mmol) emodin (1), 8g (28.6mmol) of anhydrous potassium carbonate after grinding, add 5ml (25mmol) of dimethyl sulfate, 190ml of acetone, stir for 16h, concentrate, add water and stir, filter, Wash with a small amount of cold acetone to obtain light yellow powder, 2.2g, yield 91.8%, melting point 226-228°C. 1H NMR (CDCl3 / TMS): δ: 2.46 (s, 3H), 3.95 (s, 3H,), 3.96 (s, 3H,), 3.98 (s, 3H,), 6.76 (d, J=2.5Hz, 1H), 7.09 (s, 1H,), 7.32 (d, J=2.5Hz, 1H), 7.64 (s, 1H,); IR (KBr), σ / cm-1: 3070, 2943, 2843, 1661, 1245; FAB-MS m / z (%): 313[M+H]+, elemental analysis (C 18 h 16 o 5 ): Found (calculated) / %: C 69.28 (69.23), H 5.10 (5.12).
Embodiment 3
[0025] Preparation of intermediates (3a) and (3b) mixed isomers.
[0026] Weigh 100 mg of compound (2), add 15 ml of glacial acetic acid, add 2 ml of HBr / AcOH, stir overnight at room temperature, precipitate precipitate, filter, and separate on a silica gel column (chloroform) to obtain 55 mg of orange-red crystals, yield 55%, melting point 194~ 196°C. 1H NMR (CDCl3 / TMS): δ: 2.43(s, 3H), δ 2.51(s, 3H), 3.91(s, 3H), 3.99(s, 3H), 4.03(s, 3H), 4.05(s, 3H), 6.70(d, J=2.5Hz, 1H), 6.79(d, J=2.5Hz, 1H), 6.07(s, 1H), 7.14(s, 1H), 7.30(d, J=2.5Hz, 1H), 7.46(d, J=2.5Hz, 1H), 7.57(s, 1H), 7.79(s, 1H), 13.08(s, 1H), 13.25(s, 1H); IR(KBr)σ / cm -1: 3074, 2946, 2845, 1734, 1677, 1345; FAB-MS m / z (%): 299[M+H]+, elemental analysis (C 17 h 14 o 5 ): Found (calculated) / %: C 68.55 (68.45), H 4.70 (4.73).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com