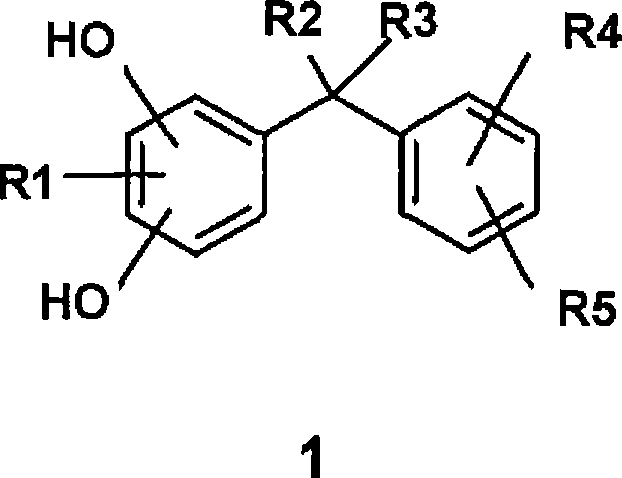
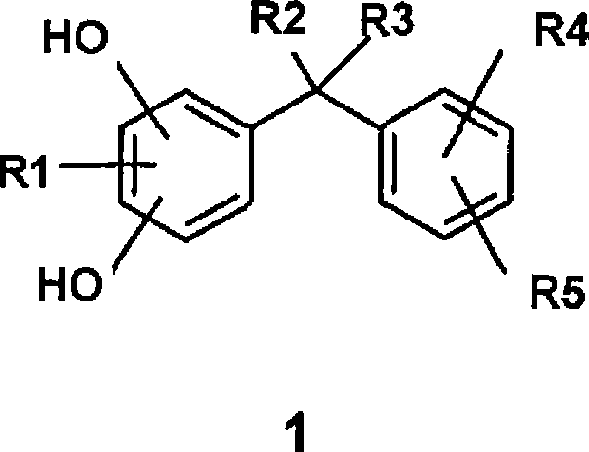
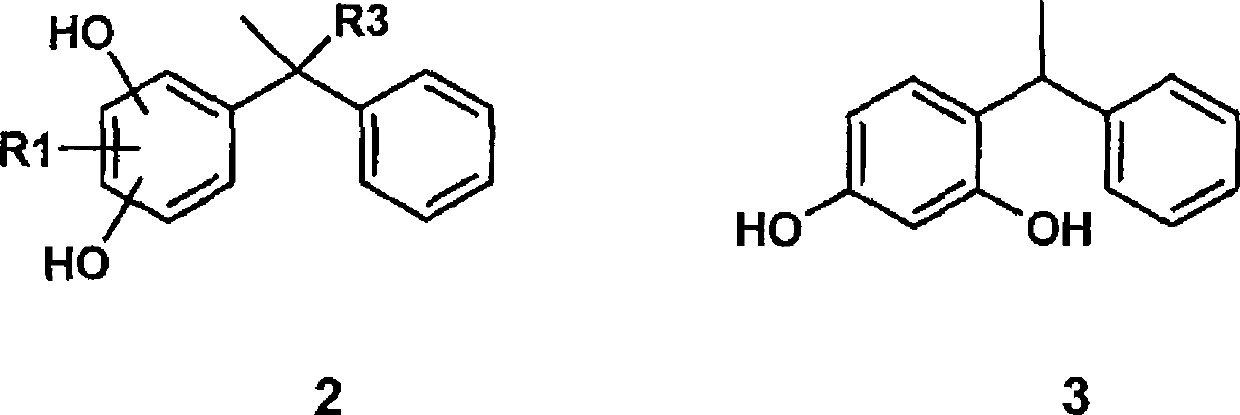
Formulations of low oil content comprising diphenylmethane derivatives
A pharmaceutical preparation and preparation technology, applied in the field of preparations containing diphenylmethane derivatives with low oil content, can solve the problem of whitening skin toxicology acceptability, skin tolerance and/or stability without predictable relationship, etc. problem, to achieve high bioavailability, effective activity, improve skin whitening activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0342] In Vivo Study of Skin Lightening Effect of Styryl Resorcinol (Formula 3)
[0343] From the following studies it was found that the diphenylmethane derivative of formula 1 has a particularly good skin whitening effect when it is applied to the skin in a formulation with a low oil phase content.
[0344] Asian volunteers (Fitzpatrick skin, type III) were treated with emulsions A-F with a size of 1.5 cm on the back 2 skin for 7 days, 2 times a day. The volunteers were irradiated with UV-A light on day 8 (4 different doses produced weak, mild, moderate and strong pigmentation, respectively). Treatment with Emulsions A-F was repeated for another 14 days. Skin color was measured visually on days 8 and 21 (L * value).
example 1
[0347] Preparation instructions :
[0348] Separately heat phases A and B to about 80°C. Add Phase B to Phase A using an Ultra-Turrax mixer and emulsify. The emulsion was stirred with a paddle stirrer until cooled, the stirring speed decreased as the temperature decreased. The pH of the emulsion was adjusted to about 5.6-5.8 with sodium hydroxide solution.
[0349] Formulation II :
[0350] The formulation is very high in water and low in lipids.
[0351] Furthermore, no customary emulsifiers but surface-modified polymers are used here. (=hydrogel cream preparation)
[0352]
Substances used
INCI name
D. emulsion
E. emulsion
f A.
Neo Pcl wssl.N
Trideceth-9
(Trideceth-9)
PEG-5
Ethylhexanoate 1.5
1.5
1.5
...
Embodiment 2
[0360] Example 2 : Study on the antioxidant capacity of styryl resorcinol (Formula 3; CARN 85-27-8; 4-(1-phenylethyl)-1,3-dihydroxybenzene) by ABTS method
[0361] Phenolic compounds generally have very good antioxidant activity. To test the degree of antioxidant potential also possessed by styryl resorcinol (Formula 3; CARN 85-27-8; 4-(1-phenylethyl)-1,3-dihydroxybenzene), the ABTS method was used to study said substance. For qualitative and quantitative evaluation of the antioxidant potential, its activity was compared with that of α-tocopherol, a highly active and versatile antioxidant.
[0362] Description of the ABTS method experiment
[0363] ABTS method is a cell-free in vitro test for evaluating antioxidant capacity (document: Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C.1999. "Antioxidant activity applying an improved ABTS radical cation decolorization assay" ; Free Radic. Biol. Med. 26:1231-7). This method exploits the inherent coloratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| spf | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com