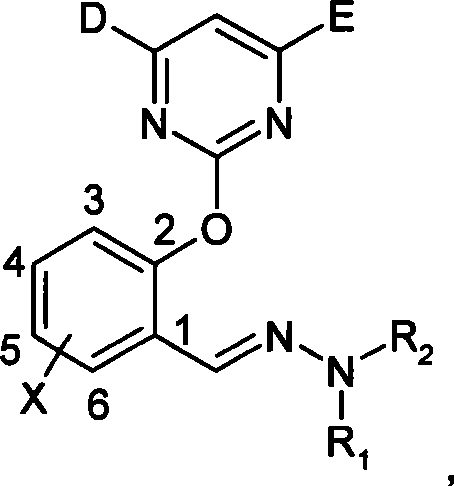
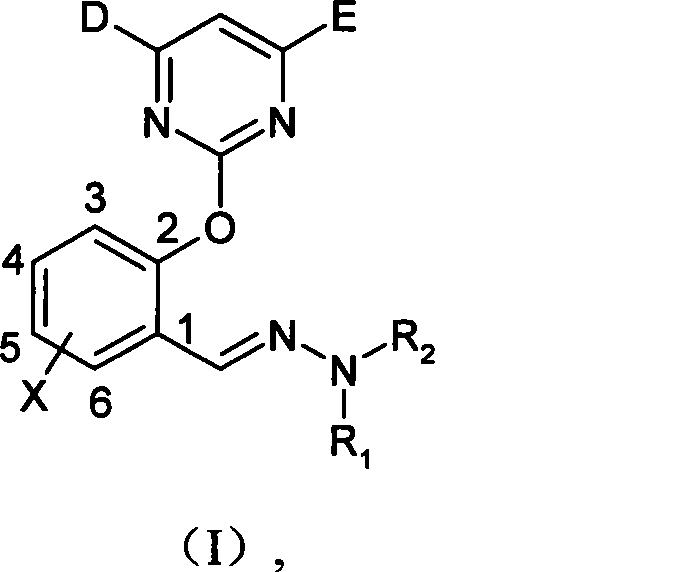
(2-pyrimidine oxygen base)benzaldehyde hydrazone compounds, preparation method and uses thereof
A kind of technology of benzaldehyde hydrazone and pyrimidineoxy, applied in the field of agrochemical herbicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of (2-pyrimidinyloxy)benzaldehyde hydrazones
[0046] Take 1 synthesis as an example:
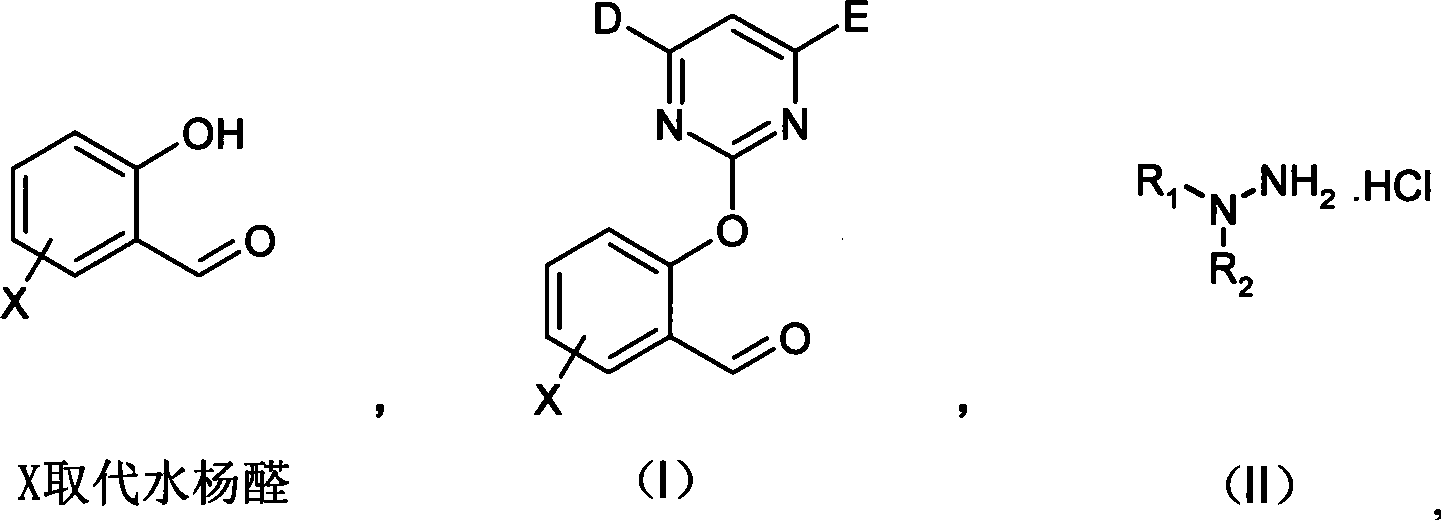
[0047] The first step: Condensation with 4,6-dimethoxy-2-thiamphenicol pyrimidine: 15.6g (0.1mol) 6-chloro salicylaldehyde, 21.8g (0.1mol) 4,6-dimethoxy - 2-thiamphenicol pyrimidine, react with 41.4g (0.3mol) of anhydrous potassium carbonate in 150mL of acetonitrile in an oil bath at 40°C for 8-9h, and control the end point of the reaction by TLC. After filtration and spin-drying, petroleum ether and ethyl acetate were recrystallized to obtain 15.4 g of pure product with a yield of 69.3%.
[0048] The second step: Condensation with pyrimidine salicylaldehyde: Dissolve 286mg (1.6mmol) m-chlorophenylhydrazine hydrochloride in a mixed solvent of ethanol and water, add dropwise 0.25ml (1.8mmol) triethylamine to dissolve m-chlorophenylhydrazine free out. Then 470mg (1.6mmol) of 6-chloropyrimidine salicylaldehyde was added to the reaction solution, and stirred at room temperat...
Embodiment 2
[0055] 2, the detailed experimental steps are the same as in Example 1: (1) Condensation with 4,6-dimethoxy-2-thiamphenicol pyrimidine: 0.1mol6-chloro salicylaldehyde in charge, 15.4g of product is obtained, yield 69.3%. (2) Condensation with pyrimidine salicylaldehyde: feed amount 1.6 mmol o-chlorophenylhydrazine hydrochloride, recrystallization from petroleum ether and ethyl acetate to obtain 230 mg of pure product, yield 36.1%.
[0056] solid
[0057] m.p.: 133.6±0.5℃
[0058] 1 H NMR (300MHz, DMSO): δ 3.74 (6H, s, OCH 3 ), 5.96 (1H, s, CH), 6.73-7.48 (7H, m, CH), 8.53 (1H, s, CH), 10.12 (1H, s, NH)
[0059] MS (ESI): 419 (M+H + ), 441 (M+Na + ), 473 (M+MeOH+Na + )
[0060] E.A. for C 19 h 16 Cl 2 N 4 o 3
Embodiment 3
[0062] 3, the detailed experimental steps are the same as in Example 1: (1) condensation with 4,6-dimethoxyl-2-thiamphenicol pyrimidine: feeding amount 0.1mol6-chloro salicylaldehyde, product 15.4g, yield 69.3%. (2) Condensation with pyrimidine salicylaldehyde: feed amount 1.6 mmol p-chlorophenylhydrazine hydrochloride, recrystallization from petroleum ether and ethyl acetate to obtain 257 mg of pure product, yield 40.3%.
[0063] 1 H NMR (300MHz, DMSO): δ 3.74 (6H, s, OCH 3 ), 5.96 (1H, s, CH), 6.71-7.44 (7H, m, CH), 8.53 (1H, s, CH), 10.14 (1H, s, NH)
[0064] MS (ESI): 419 (M+H + ), 441 (M+Na + ), 473 (M+MeOH+Na + )
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com