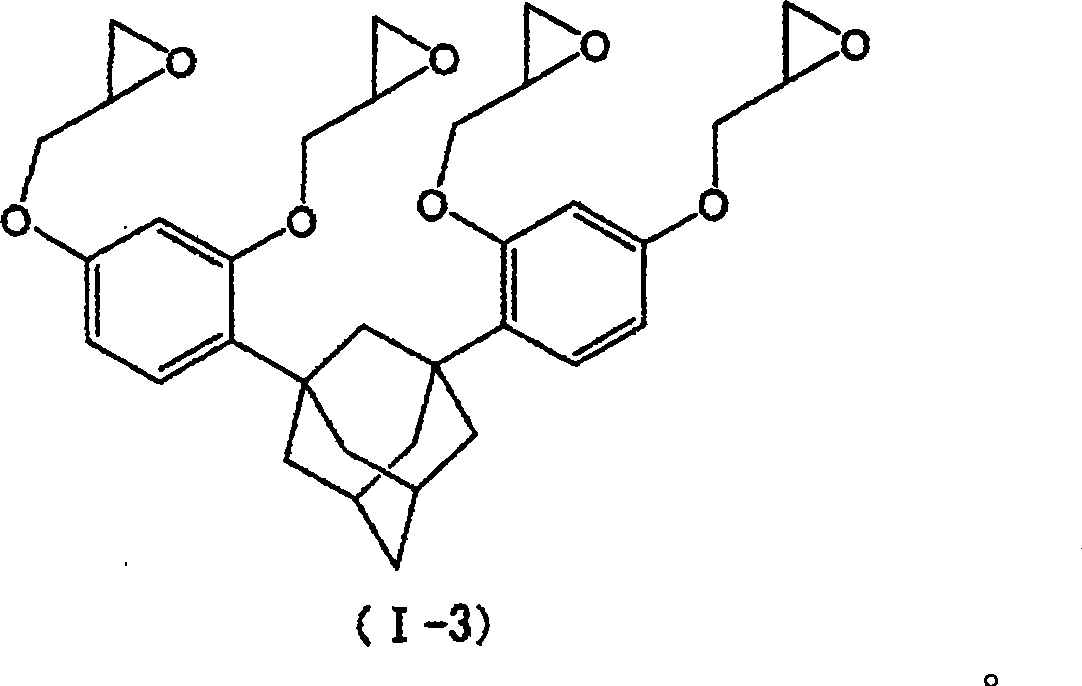
Adamantane derivative, resin composition containing same, and optoelectronic member and sealing agent for electronic circuit using those
一种树脂组合物、金刚烷的技术,应用在有机化合物的制备、用于光机械设备的光敏材料、光学等方向,能够解决电子电路介电常数高、很难耐热性充分、黄变等问题,达到吸水性优异、透明性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
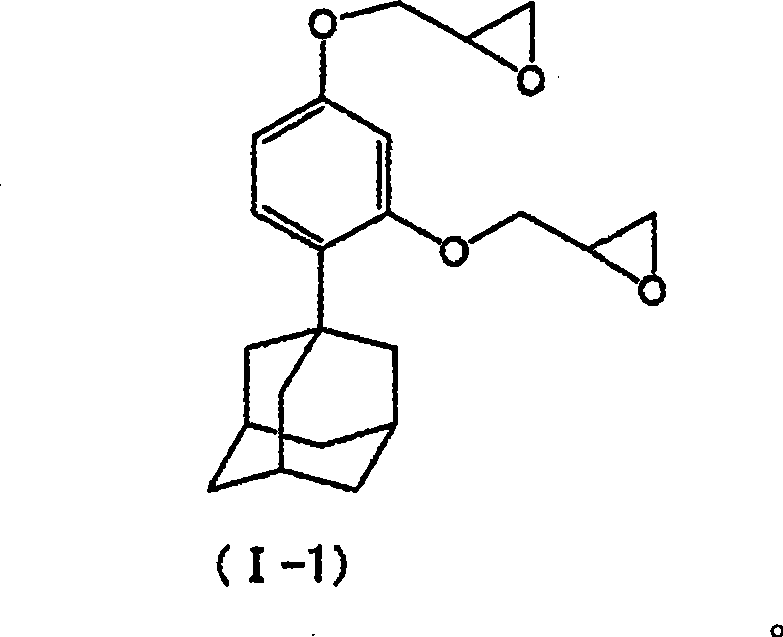
[0137] Embodiment 1 [1-(2,4-diglycidyloxyphenyl) adamantane [synthesis of formula (I-1)]] (1) 1-(2,4-dihydroxyphenyl) adamantine Synthesis of alkanes
[0138] Add 28.1g (0.18mol) 1-adamantanol, 8.8g (0.05mol) p-toluenesulfonic acid monohydrate and 35mL Ethanol for nitrogen replacement. 39.6 g (0.36 mol) of resorcinol was added thereto. This was added to a 90° C. oil bath, and heated and stirred for 3 hours. The reaction solution was cooled, then 200 mL of pure water was added, stirred for 30 minutes, and then filtered to obtain a solid. The solid was dried under reduced pressure, then recrystallized with methanol / toluene mixed solvent, and the obtained crystal was taken out while washing with toluene, and dried under reduced pressure to a constant weight to obtain the target compound (yield 86%).
[0139] Through nuclear magnetic resonance spectroscopy ( 1 H-NMR, 13 C-NMR) to identify the compound.
[0140] NMR spectroscopy was performed using DMSO-d 6 As a solvent, it...
Embodiment 2
[0149] Example 2 [Synthesis of 1-(2,3,4-trihydroxyphenyl)adamantane [the above formula (II-1)]]
[0150]In Example 1 (1), the resorcinol was changed to 45.3g (0.36mol) pyrogallol, except that the same operation was performed as in Example 1 (1), to obtain the target compound (yield 78%) .
[0151] Through nuclear magnetic resonance spectroscopy ( 1 H-NMR, 13 C-NMR) to identify the compound.
[0152] NMR spectroscopy was performed using DMSO-d 6 As a solvent, it measured with JNM-ECA500 manufactured by JEOL Ltd.
[0153] 1 H-NMR (500MHz): 1.70(s, 6H), 2.02(s, 9H), 6.20(d, IH), 6.36(d, 1H), 7.77(br, 1H), 8.20(br, 1H), 8.84 (br,1H)
[0154] 13 C-NMR (125MHz): 28.46, 35.52, 36.68, 40.30, 105.49, 115.41, 127.27, 132.56, 143.84, 145.36
Embodiment 3
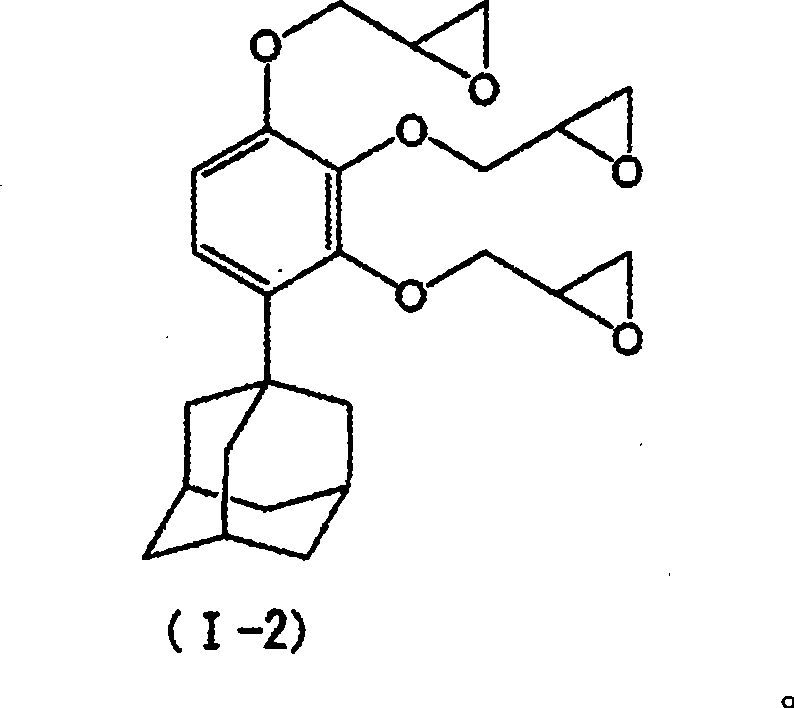
[0155] Example 3 [Synthesis of 1-(2,3,4-triglycidyloxyphenyl)adamantane [the above formula (I-2)]]
[0156] In embodiment 1 (2), change 1-(2,4-dihydroxyphenyl) adamantane into 20.5g (0.08mol) 1-(2,3,4-trihydroxyl) synthesized in embodiment 2 Phenyl) adamantane, the usage-amount of epichlorohydrin is changed into 80g (0.86mol), the usage-amount of sodium hydroxide is changed into 12.2g (0.30mol), carry out similarly with embodiment 1 (2) except this Operation to obtain the target compound (yield 90%). The LC purity of this compound was 92% (92% of the monomer and the rest were oligomers of the target compound).
[0157] Through nuclear magnetic resonance spectroscopy ( 1 H-NMR, 13 C-NMR) to identify the compound.
[0158] NMR spectroscopy was performed using DMSO-d 6 As a solvent, it measured with JNM-ECA500 manufactured by JEOL Ltd.
[0159] 1 H-NMR (500MHz): 1.76(s, 6H), 2.04(s, 9H), 2.68(m, 3H), 2.85(m, 3H), 3.36(m, 3H), 3.84(m, 3H), 4.28 (m, 3H), 6.53(d, 1H), 6.68(d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| cure temperature | aaaaa | aaaaa |
| visible light transmittance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com