Carbpenem penicillin ertapenem intermediate, and preparation and use thereof
A technology of carbapenems and ertapenem, which is applied in the field of organic compound synthesis, can solve problems such as difficult industrial production, high equipment requirements, and harsh conditions, and achieve the effect of simple operation, simple experimental conditions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
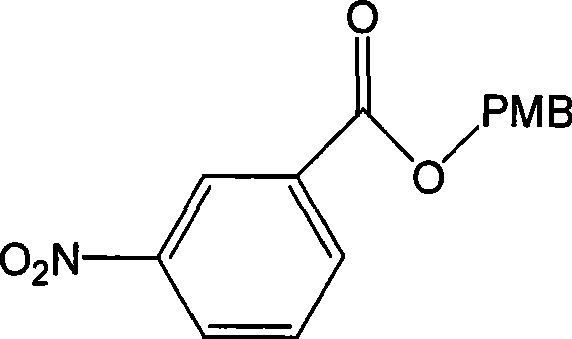
[0034] Reference Examples Preparation of Formula II Compounds
[0035] Formula I compound (26.5g, 157.9mmol) in CH 2 Cl 2 350ml, add NEt 3 (32g, 316.1mmol), 4-methoxybenzyl chloride (PMBCl) (44.4g, 283.6mmol) was added dropwise at room temperature, the temperature was raised to 45°C, and the reaction was carried out for 3 hours. use CH 2 Cl 2 and water extraction, combined CH 2 Cl 2 layer with anhydrous NaSO 4 Dry, filter, and concentrate the filtrate to obtain a light yellow solid (Formula II) (41.5 g, yield 91.5%, purity >99%).
Embodiment 1
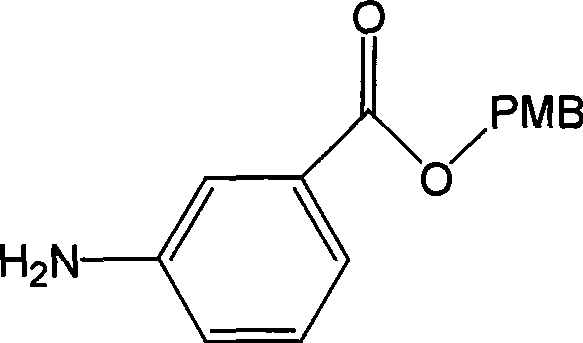
[0036] The synthesis of embodiment 1 3-aminobenzoic acid 4-methoxybenzyl ester (formula III)
[0037] Compound shown in formula II (10.00g, 34.81mmol) in SnCl 2· 2H 2 O (39.33g, 174.05mmol) in 70ml of ethanol, heated up to 50°C, and reacted for 30min. Use 8wt% NaOH alcohol solution to adjust pH=9, filter, extract with ether and water, combine the ether layers, anhydrous NaSO 4 Dry, filter, and concentrate the filtrate to obtain a light yellow solid (Formula III) (8.10 g, 90.5%). Melting point: 45-47°C.
[0038] NMR (CDCl 3 )δ: 3.77(s, 3H); 5.25(s, 2H); 6.80(m, 1H); 6.89(m, 2H); 7.16(m, 1H); 7.33(m, 2H); ); 7.43 (m, 1H)
[0039] MS(CI): 258[M+H]
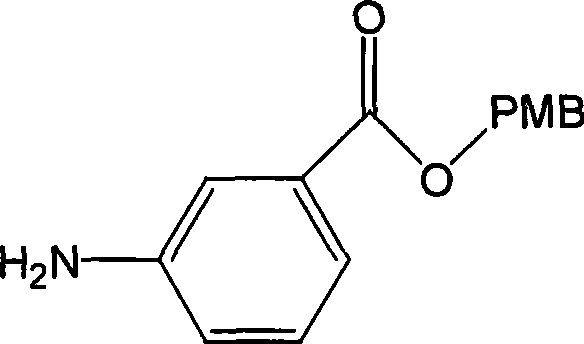
Embodiment 2
[0040] The synthesis of embodiment 2 3-aminobenzoic acid 4-methoxybenzyl ester (formula III)
[0041] Compound shown in formula II (40.93g, 142.47mmol) in SnCl 2· 2H 2 O (161.0g, 712.37mmol) in 300ml of ethanol, heated to 50°C, and reacted for 50min. Adjust the pH to 11 with 15% NaOH alcohol solution, extract with ether and water, combine the ether layers, anhydrous NaSO 4 Dry, filter, and concentrate the filtrate to obtain a light yellow solid (Formula III) (33.84 g, 92.3%). Melting point: 50-52°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com