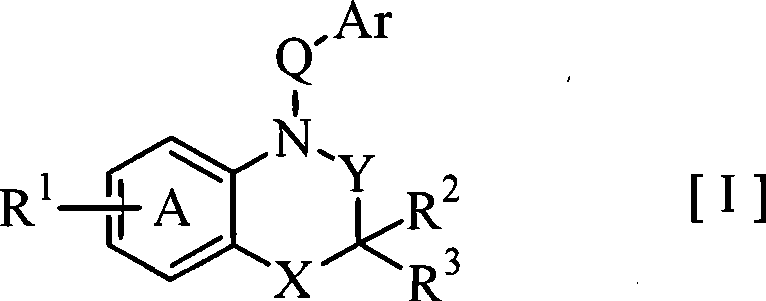
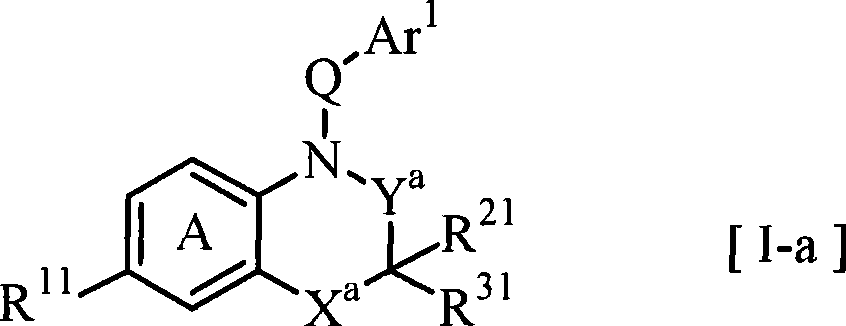
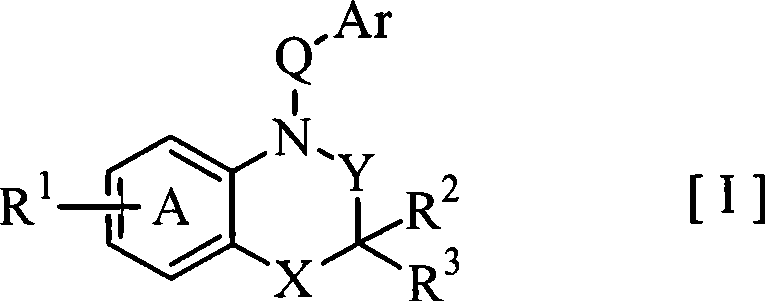
Benzoxazines and related nitrogen-containing heterobicyclic compounds useful as mineralocorticoid receptor modulating agents
A kind of technology of compound and benzene ring, applied in the field of nitrogen-containing heterocyclic compounds, can solve the problems such as not reporting benzoxazine-sulfonamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0485] To 7-amino-2,2-dimethyl-4-phenyl-2H-1,4-benzoxazin-3(4H)-one (obtained in reference example 1(3)) under ice-cold conditions To a solution of compound, 50 mg) in chloroform (2 mL), methanesulfonyl chloride (22 μL) and pyridine (30 μL) were successively added dropwise and the mixture was stirred at room temperature for 18 hours. To the reaction mixture was added saturated sodium bicarbonate solution, and the mixture was extracted with chloroform. The organic layer was washed sequentially with water, 10% HCl solution and brine, dried over sodium sulfate and concentrated in vacuo. The resulting residue was purified by column chromatography on NH-silica gel (solvent; n-hexane / ethyl acetate=1 / 1→ethyl acetate) to give N-(2,2-dimethyl-3-oxo-4 -Phenyl-3,4-dihydro-2H-1,4-benzoxazin-7-yl)methanesulfonamide (55 mg), a colorless powder. MS(APCI) m / z: 347[M+H] +
Embodiment 2 to 38
[0487] The corresponding materials were treated in the same manner as described in Example 1 to obtain the compounds shown in Tables 1 to 8 below.
[0488] Table 1
[0489]
[0490] Table 2
[0491]
[0492]
[0493] table 3
[0494]
[0495] Table 4
[0496]
[0497]
[0498] table 5
[0499]
[0500] Table 6
[0501]
[0502] Table 7
[0503]
[0504] Table 8
[0505]
Embodiment 39
[0507] 7-Amino-4-(4-fluorophenyl)-2,2-dimethyl-2H-pyrido[3,2-b][1,4]oxazin-3(4H)-one (the compound obtained in Reference Example 7(4), 113 mg) was treated in the same manner as described in Example 1 to give N-[4-(4-fluorophenyl)-2,2-dimethyl -3-Oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazin-7-yl]methanesulfonamide (98 mg) as colorless crystals.
[0508] MS(APCI) m / z: 366[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com