Use of CR-1 siRNA in treating myocardial hypertrophy induced by AngII
A technology of cardiac hypertrophy and cr-1siRNA, which can be used in gene therapy, cardiovascular system diseases, genetic material components, etc., and can solve the problems that have not been seen before.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
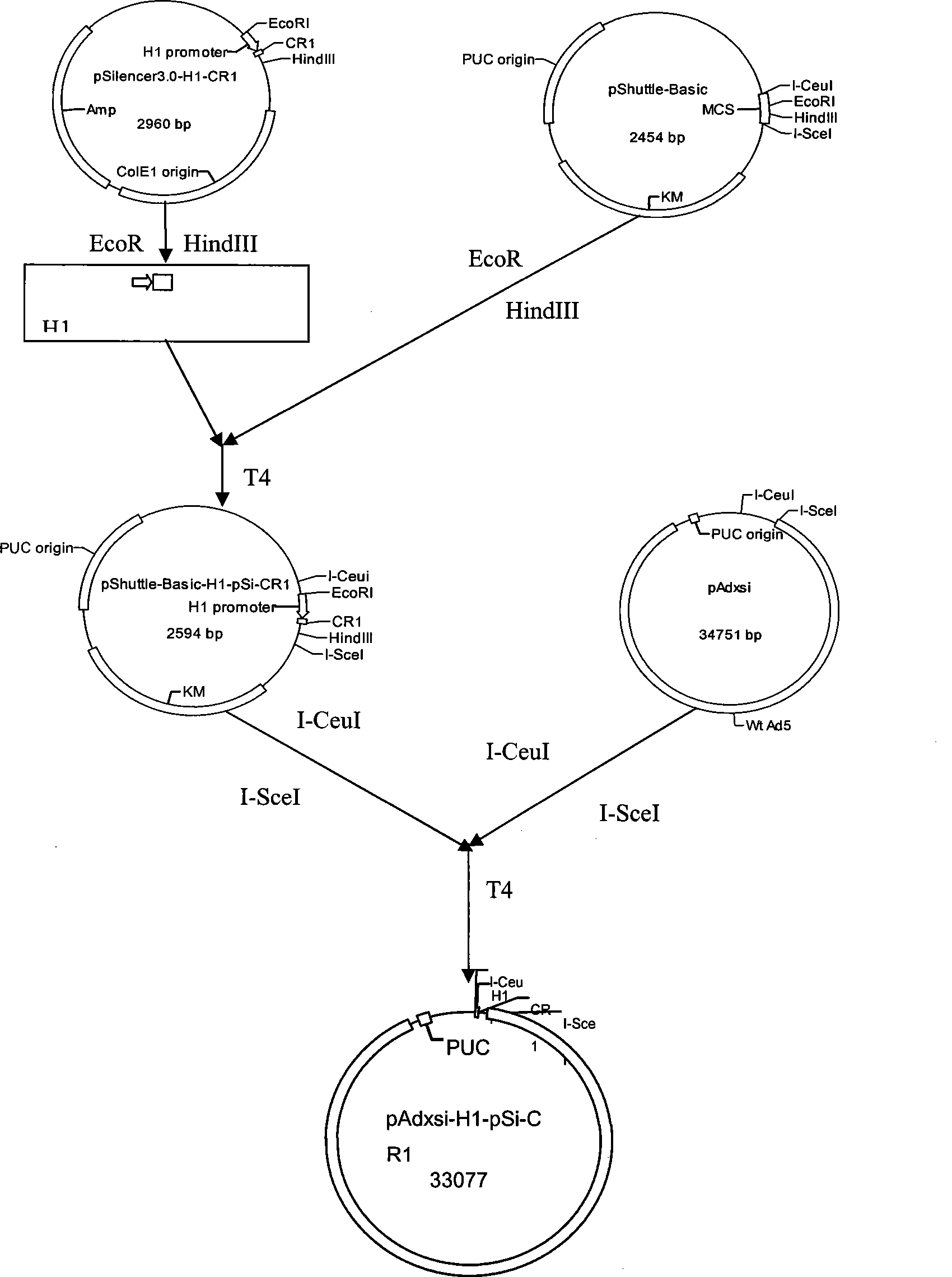
[0035] Construction of CR-1siRNA adenovirus recombinant vector
[0036] This experiment uses the vector pSilencer3.0-H1 (Ambion Company) which has H1 promoter and can express shRNA efficiently in mammalian cells. According to the cDNA sequence of CR-1 (AF417001), design and synthesize the positive and negative double-stranded DNA oligonucleotide fragments targeting the target sequence AACAAGGCTTCTCATAACAGG,
[0037] 5′-GATCCCGCAAGGCTTCTCATAACAGGTTCAAGAGACCTGTTATGAGAAGCCTTG
[0038] TTTTTTGGAAA-3′
[0039] 5′-AGCTTTTCCAAAAAACAAGGCTTCTCATAACAGGTCTCTTGAACCTGTTATGAG
[0040] AAGCCTTGCGG-3′
[0041] After the two DNA fragments were annealed, they were ligated into the pSilencer3.0-H1 vector through the BamHI and HindIII sites to construct the pSilencer3.0-H1-CR-1siRNA recombinant vector; the H1-CR-1siRNA obtained by digestion with EcoRI / HindIII The (0.16kb) fragment is connected with the pShuttle-Basic shuttle vector (Northsay Genetics Co., Ltd.) to obtain the pShuttle-Basic-H...
Embodiment 2
[0042] Replication of mouse AngII induced cardiac hypertrophy model
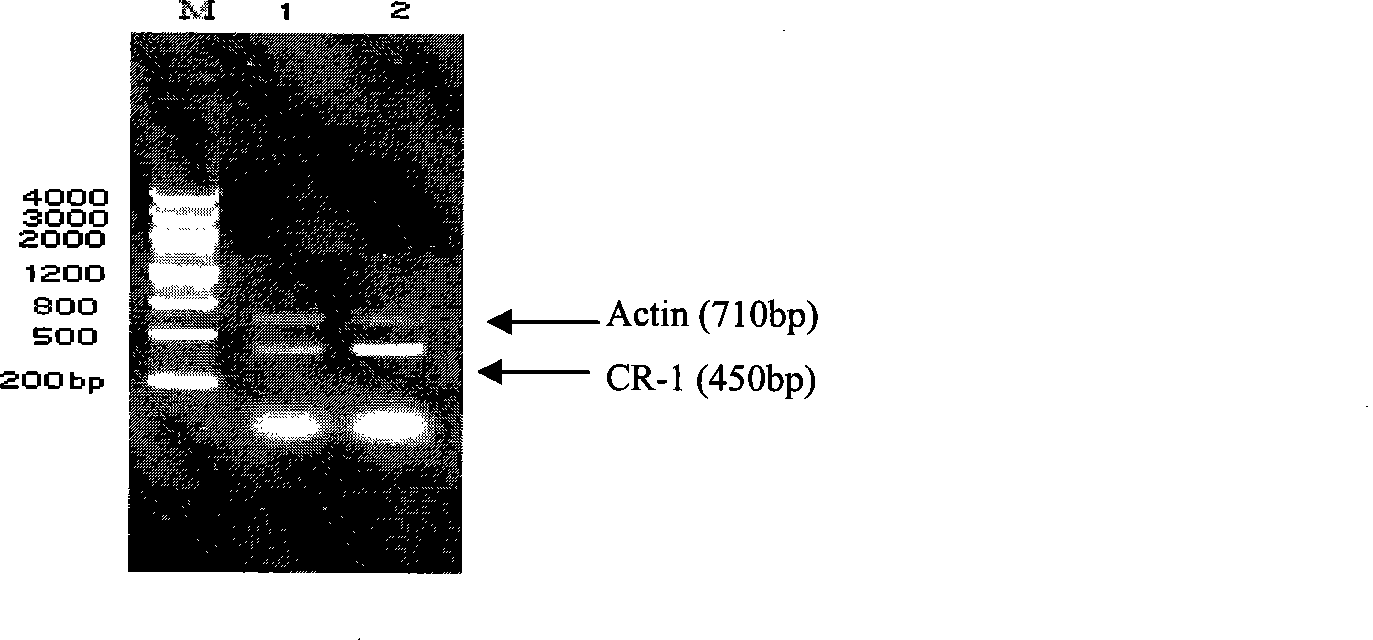
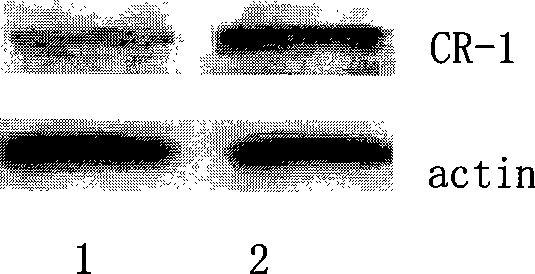
[0043] Eighteen clean-grade 8-week-old C57BL / 6 mice (purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences) were randomly divided into three groups A, B, and C on the third day after entering the laboratory, with 6 mice in each group. Group A was then randomly selected for surgery. After the mice were anesthetized with 2.5% tribromoethanol (14uL / g), a micropump (Alzet Company Model 2002) prepacked with AngII (2.5mg / (kg. The micropump treatment with prepacked solvent medium (PBS) was used as the control, and group C was set as the non-pump control group. Ultrasound (Aloka ultrasonic instrument SSD-5500, 7.5MHz probe) was used to detect the morphological changes of the mouse myocardium, and reverse transcription polymer chain reaction (RT-PCR) and Western blot were used to detect the expression of CR-1 gene (Table 1 ), ( figure 2 , 3). The results showed that the myoca...
Embodiment 3
[0048] CR-1siRNA adenovirus recombinant vector is introduced into mouse AngII-induced myocardial hypertrophy model
[0049] Take 36 clean-grade 8-week-old C57BL / 6 mice (purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences), and on the third day after entering the laboratory, they were randomly divided into three series A, B and C. There are three experimental groups with 6 mice in each group. See Table 2 for series and experimental groupings.
[0050] Table 2 CR-1siRNA adenovirus recombinant vector introduced into AngII to treat the experimental grouping of mice
[0051]
[0052] The mice in groups 3, 6, and 9 were injected with siRNA-CR1 (2 X 10 9 pfu / μl, 200μl / d), groups 2, 5, and 8 were injected with adenovirus vector control (2 X 10 9 pfu / μl, 200μl / d), for a total of 13 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com