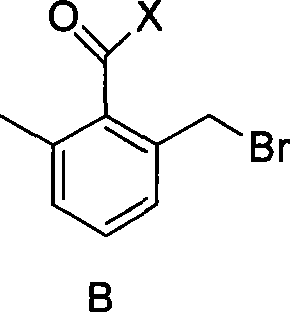
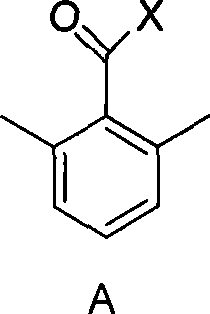
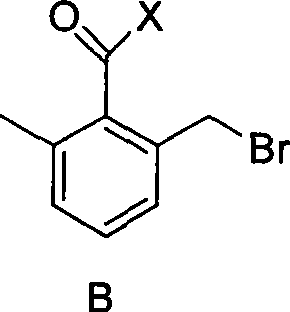
2-bromomethyl-6-methyl benzoyl chloride/bromium and preparation method thereof
A technology of methylbenzoyl chloride and dimethylbenzoyl, which is applied in the field of new compounds and their preparation, can solve the problem of inability to selectively synthesize bromomethylbenzene compounds, and achieve great economic value, yield and purity High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2-bromomethyl-6-methylbenzoyl chloride
[0020] At room temperature, add 2,6-dimethylbenzoyl chloride (0.1mol), dichloromethane (50mL), bromosuccinamide (NBS) (0.08mol), azoisobutylcyanide (AIBN ) (0.0002mol), stirred evenly and then stirred at 40°C until the peak area of the product was no longer increased by GC tracking (4h). After the reaction was finished, dichloromethane was distilled off, and then the product 2-bromomethyl-6-methylbenzoyl chloride was obtained by distillation with a yield of 77% and a purity of 98.2% (GC).
Embodiment 2
[0021] Example 2 2-bromomethyl-6-methylbenzoyl chloride
[0022] At room temperature, add 2,6-dimethylbenzoyl chloride (0.1mol), carbon tetrachloride (40mL), NBS (0.09mol), benzoyl peroxide (BPO) (0.0002mol) to the reaction flask, Azoisobutyrocyanide (AIBN) (0.0002mol), stirred evenly and then stirred at 70°C until the peak area of the product no longer increased after GC tracking (5h). After the reaction, the carbon tetrachloride was evaporated, and then the product 2-bromomethyl-6-methylbenzoyl chloride was distilled with a yield of 87% and a purity of 98.0% (GC).
Embodiment 3
[0023] Example 3 2-bromomethyl-6-methylbenzoyl chloride
[0024] At room temperature, add 2,6-dimethylbenzoyl chloride (0.1mol), 1,2-dichloroethane (60mL), bromohydantoin (0.095mol), AIBN (0.0005mol) into the reaction flask, After stirring evenly, stir at 70°C until the peak area of the product does not increase after GC tracking (4h). After the reaction was completed, 1,2-dichloroethane was evaporated, and then the product 2-bromomethyl-6-methylbenzoyl chloride was obtained by distillation with a yield of 89% and a purity of 98.6% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com