Process of forming a polyol
A polyol, form technology, applied in chemical instruments and methods, sugar derivatives, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
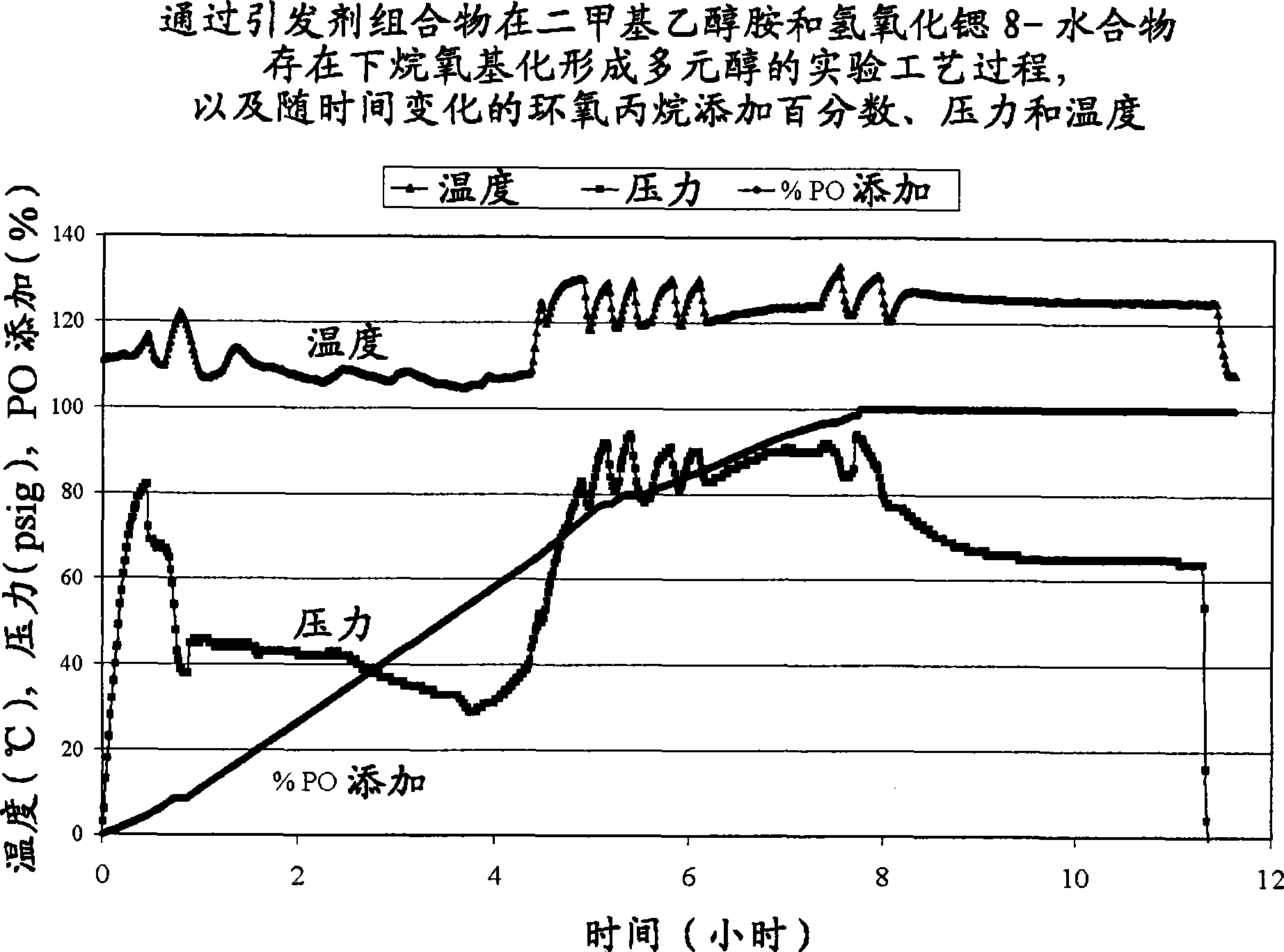
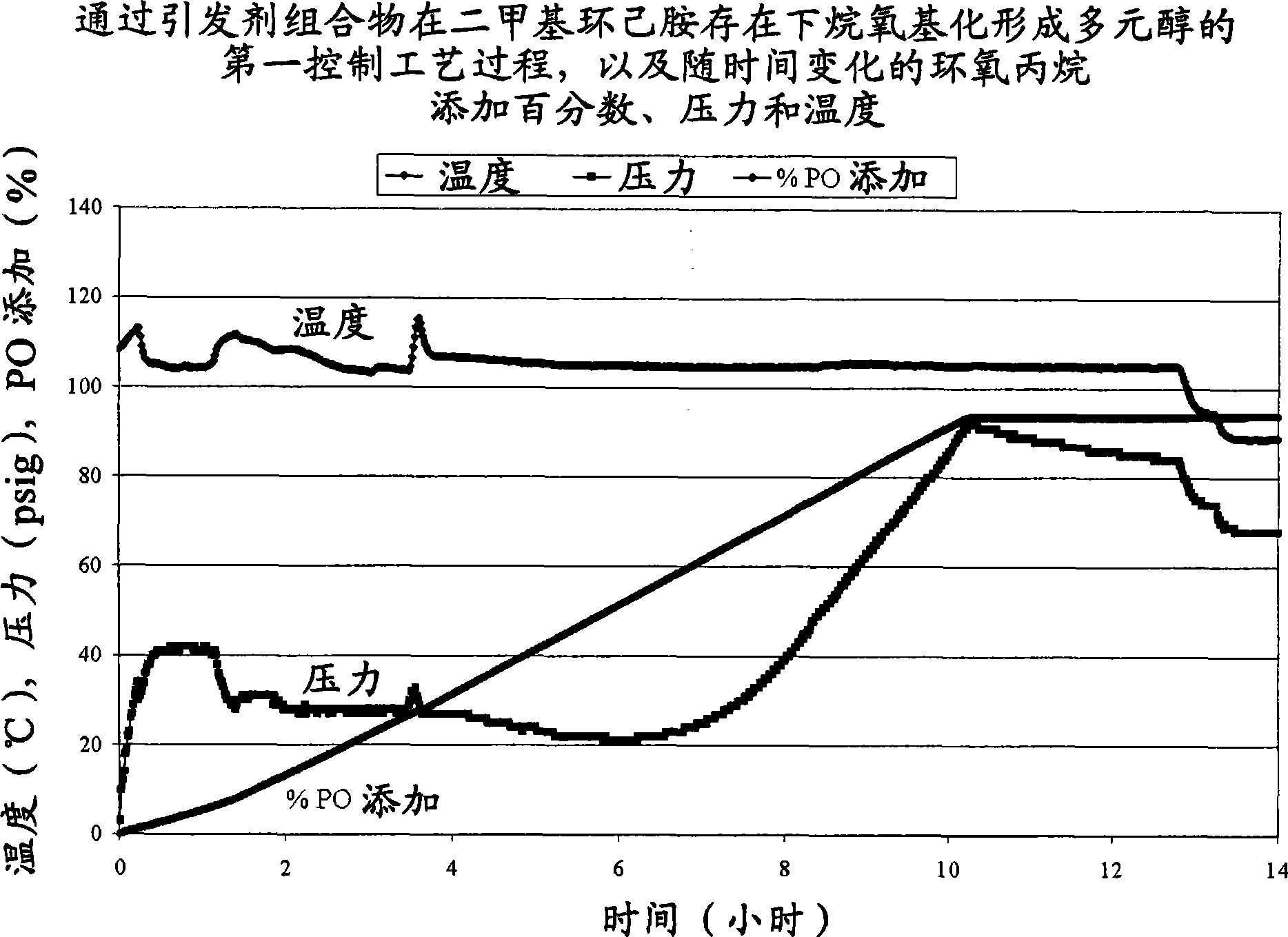
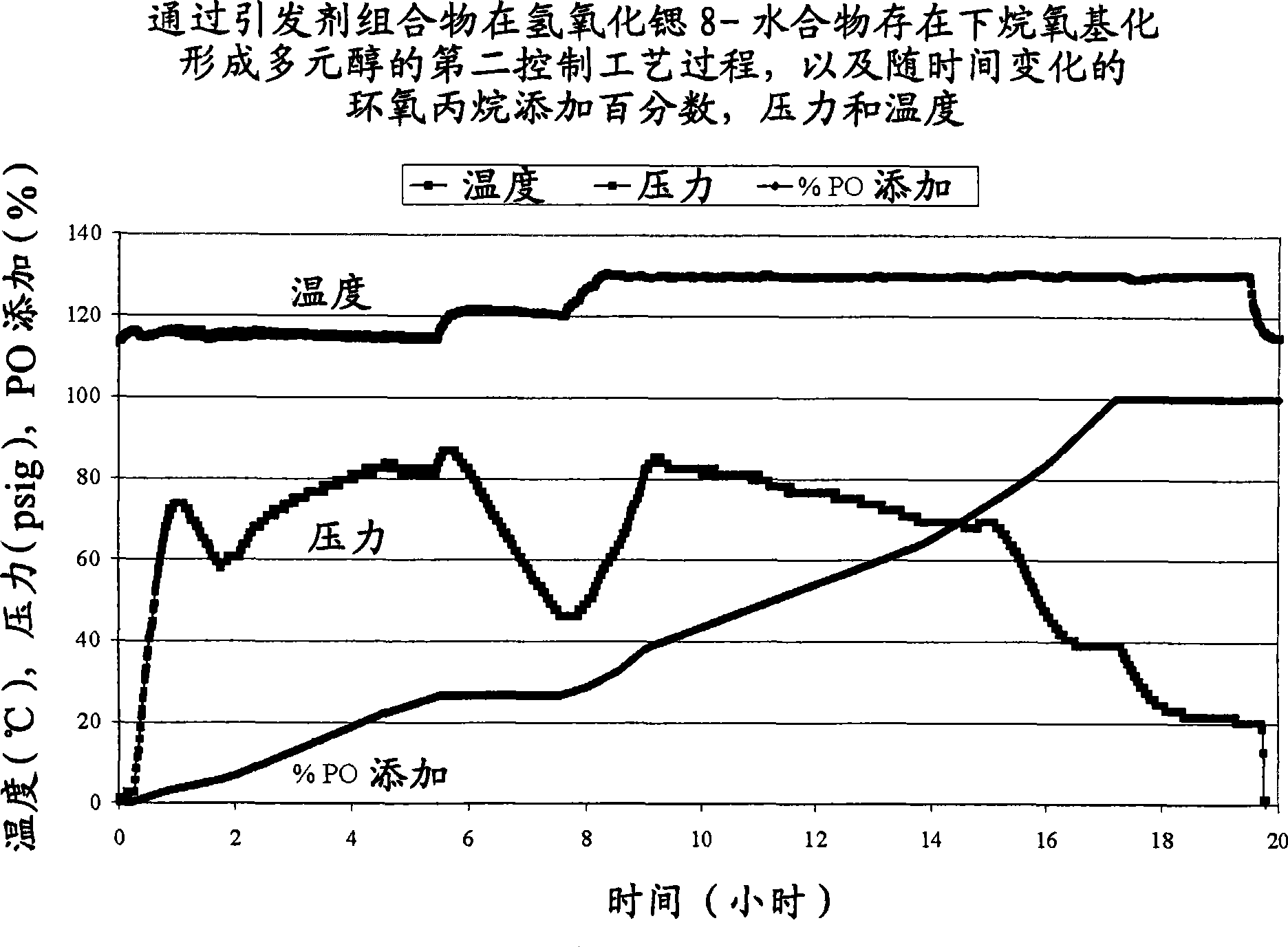
[0030] Polyol 1 is formed according to the process of the invention in the presence of an alkaline earth metal hydroxide and a first amine. Two comparative polyols, Comparative Polyol 1 and 2, were not formed by the process of the present invention. Comparative Polyol 1 was formed by reacting an initiator composition and an alkylene oxide in the presence of a second amine without an alkaline earth metal hydroxide. Comparative Polyol 2 was formed by reacting an initiator composition and an alkylene oxide in the presence of an alkaline earth metal hydroxide without the first or second amine. Specific amounts of the initiator composition, alkylene oxide, alkaline earth metal hydroxide, first amine, and second amine are listed in Table 1 below. The reaction times, formulated hydroxyl values and experimental hydroxyl values for Polyol 1 and Comparative Polyols 1 and 2 are also listed in Table 1. Unless otherwise stated, all components of Polyol 1 and Comparative Polyol 1 are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com