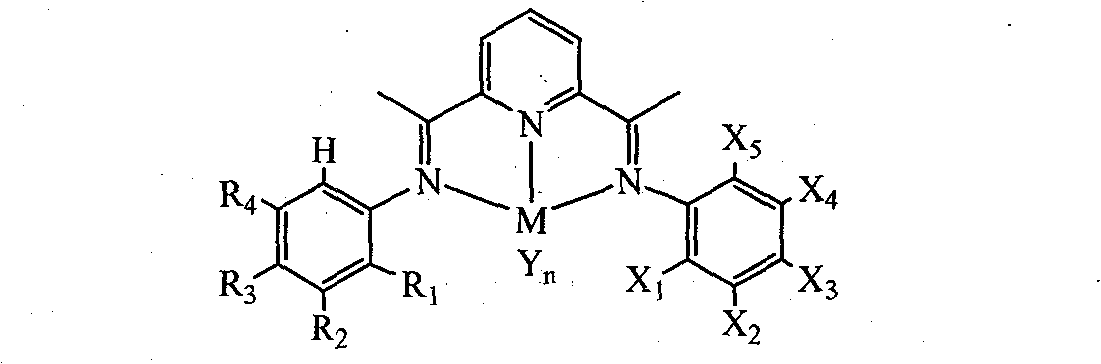
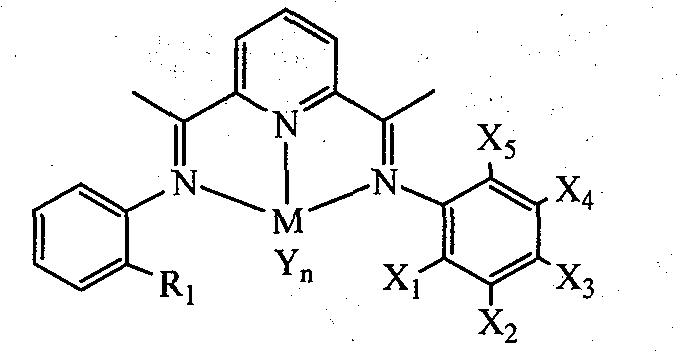
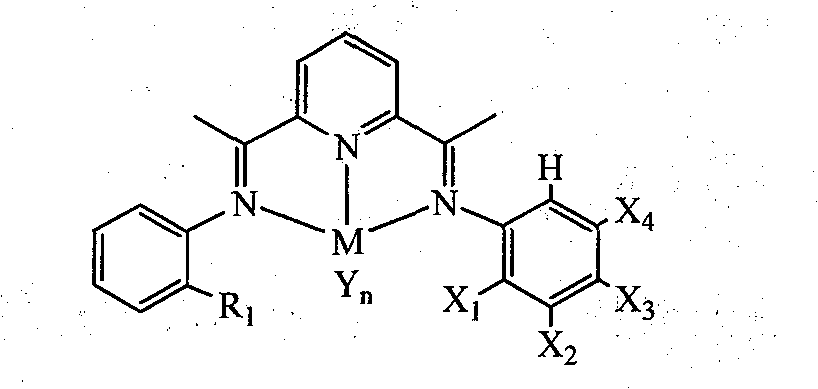
Unsymmetrical bis(imino)pyridines iron and cobalt complexes containing halogen, preparation method and use
A technology of pyridine bisimide and cobalt complexes, which is applied in the field of fine chemicals, can solve the problems of decreased added value competitiveness and high content of oligomer C4, and achieve the effects of improved selectivity, high oligomerization activity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 2-Acetyl-6-{1-[(2-methylphenyl)imine]ethyl}pyridine (D1)
[0040]
[0041] Take 1.64g of DAP and 40ml of methanol in a 100ml flask, add 4-5 drops of glacial acetic acid, take another 1.0g of o-methylaniline in a dropping funnel, add 20ml of methanol, slowly drop the methanol solution of o-methylaniline In the flask containing DAP, react under ultrasonic conditions while adding dropwise, the temperature is 30°C, and the ultrasonic power is 500W. After the reaction was terminated, the solvent was distilled off, and the concentrate was crystallized in methanol to obtain 1.70 g of a yellow solid with a yield of 71.5%. m.p50-52°C. IR (KBr, cm -1 ): 3062, 2966, 2926, 1695 (C=O), 1642 (C=N), 1577, 1480, 1359, 1311, 1246, 1224, 1109, 1078, 824, 786, 747. 1 HNMR (400MHz, CDCl 3 ): δ8.52(d, 1H, Py-Hm), 8.12(d, 1H, Py-Hm), 7.93(t, 1H, Py-Hp), 7.23(m, 2H, ph), 7.05(t, 1H, Ph), 6.68 (d, 1H, Ph), 2.79 (s, 3H, O=C-CH 3 ), 2.35(s, 3H, N=C-CH 3 ), 2.12 (s, 3H, PhCH 3 )
Embodiment 2
[0043] 2-Acetyl-6-{1-[(2-ethylphenyl)imine]ethyl}pyridine (D2)
[0044] The preparation method was the same as that of D1 to obtain a yellow solid with a yield of 56.9%. m.p.43-46℃.IR(KBr, cm -1 ): 2970, 2926, 1700 (C=O), 1643 (C=N), 1481, 1444, 1364, 1310, 1226, 1115, 1084, 1047, 819, 787, 743. 1 HNMR (400MHz, CDCl 3 ): δ8.52(d, 1H, Py-Hm), 8.12(d, 1H, Py-Hm), 7.95(t, 1H, Py-Hp), 7.23(m, 2H, ph), 7.09(t, 1H, Ph), 6.66 (d, 1H, Ph), 2.79 (S, 3H, O=C-CH 3 ), 2.49 (q, 2H, Ph-CH 2 -), 2.37(s, 3H, N=C-CH 3 ), 1.16(t, 3H, Ph-C-CH 3 )
Embodiment 3
[0046] 2-{1-[(2-methylphenyl)imino]ethyl}-6-{1-[(2-chlorophenyl)imino]ethyl}}pyridine
[0047]
[0048] In a 50ml Schlenk, add 0.31g (1.25mmol) 2-acetyl-6-{1-[(2-methylphenyl) imino] ethyl} pyridine (D1), 0.185g (1.45mmol ) 2-chloroaniline, 10ml toluene, 1g molecular sieve and 0.2gSi-Al catalyst, N 2 Under protection, stir and react at 35-40°C for 4 days, filter to remove the molecular sieve and Si-Al catalyst, wash the solid with toluene 3-4 times, combine the filtrate and evaporate the solvent toluene with a rotary evaporator, and the remaining concentrate (primary product) Recrystallized with methanol to obtain a yellow solid 0.107g, yield 23.7%. m.p80-83 ° C. IR (KBr, cm -1 ): 3062, 3014, 2969, 2921, 1644, 1575, 1465, 1363, 1316, 1220, 1115, 1066, 817, 779, 736. 1 HNMR (400MHz, CDCl 3 ): δ8.42(d, 2H, Py-Hm), 7.90(t, 1H, Py-Hp), 7.20-7.30(m, 4H, ph), 7.05(m, 2H, Ph), 6.85(m, 1H, Ph), 6.68(d, 1H, Ph), 2.38(s, 3H, N=C-CH 3 ), 2.34(s, 3H, N=C-CH 3 ), 2.13 (s, 3H, PhCH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com