Method for synthesizing emtricitabine intermediate
A synthesis method, the technology of oxathiolane, is applied in the field of synthesis of emtricitabine intermediates, which can solve problems affecting the health and safety of operators, high toxicity of raw materials, environmental pollution, etc., and achieve low production cost, The effect of simplifying the reaction steps and saving production costs
Active Publication Date: 2010-12-22
JIANGSU COBEN PHARMA CO LTD +1
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In this reaction, due to the use of thionyl chloride as the chlorination reagent, the raw materials are highly toxic. At the same time, a large amount of sulfur dioxide waste gas is generated during the reaction process, which seriously corrodes the equipment and seriously pollutes the environment, affecting the health and safety of operators.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
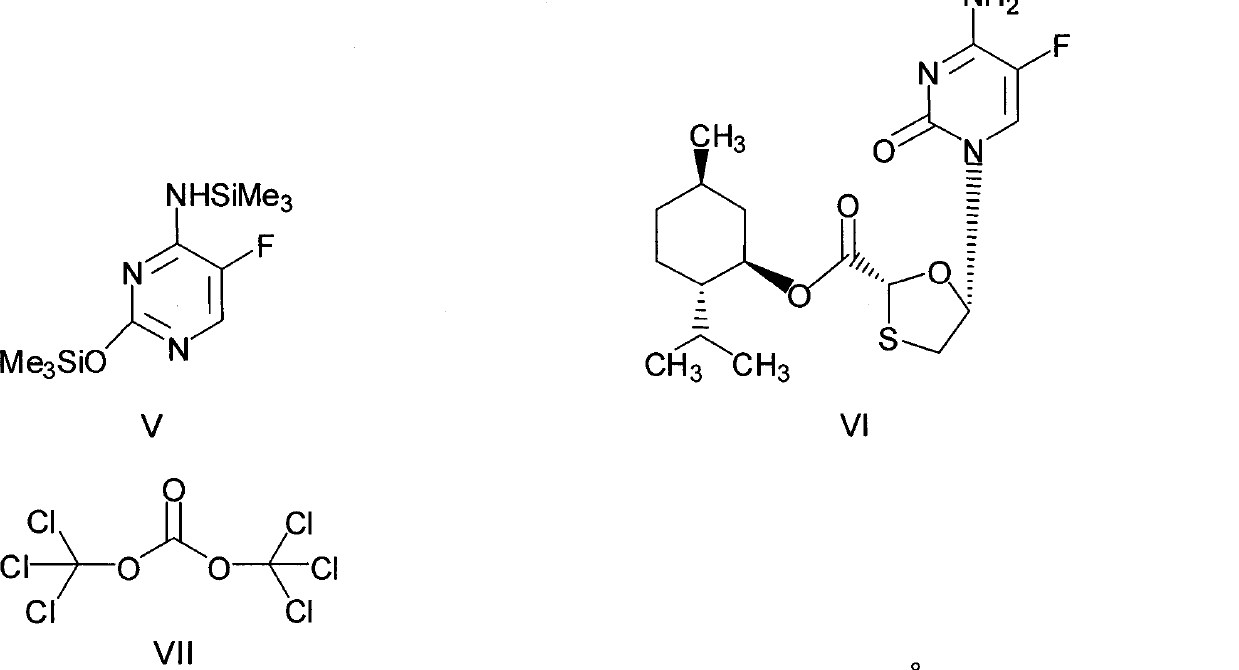
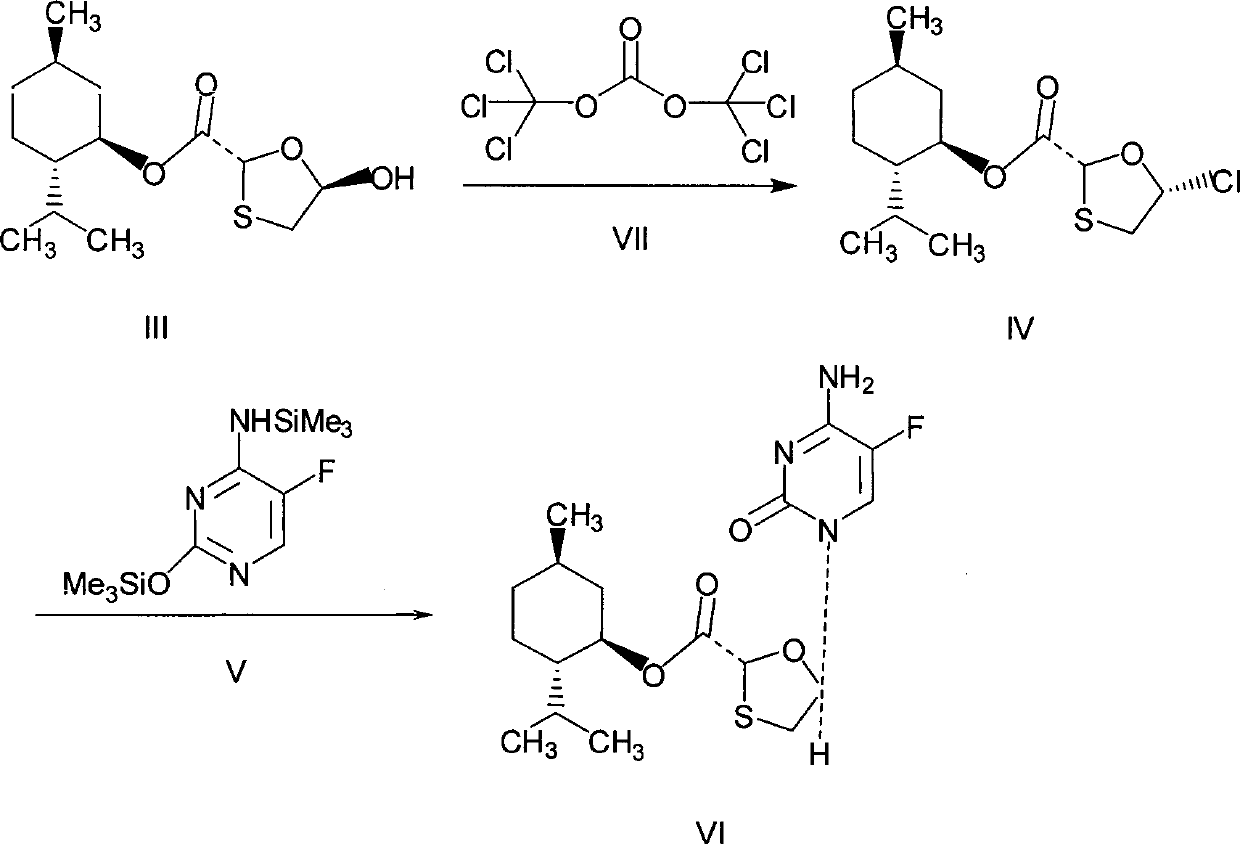
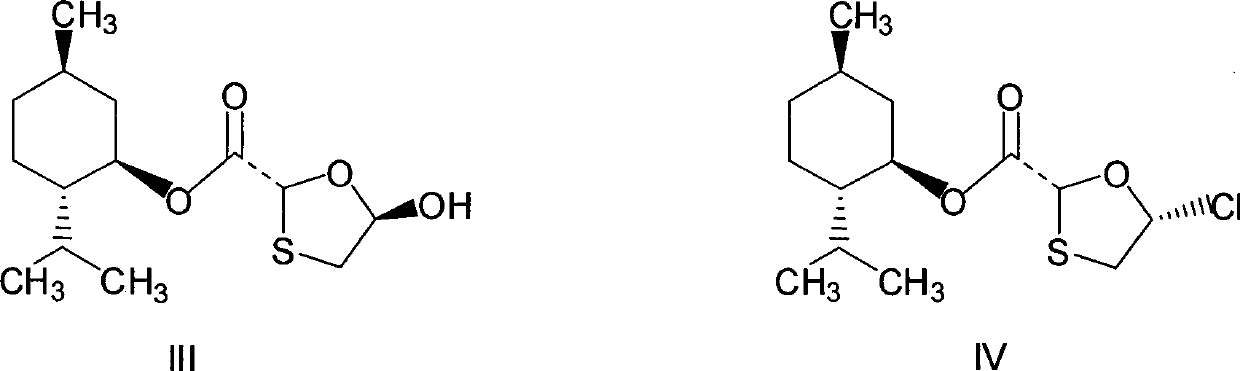
The invention discloses a synthetic method of emtricitabine intermediates, namely, (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (VI), (2R, 5S)-5-hydroxy-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (III) is taken as raw material to obtain chloro compounds having the structural formula of (IV) by chlorination reaction, then the chloro compounds (IV) are treated with condensation and hydrolyzation reactions with N, O-bis(trimethoxy)5-flurocytosin having the structural formula of (V), thus obtaining the (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester (VI). The invention uses bis(trichloromethyl) carbonic ester to replace the thionyl chloride in the prior art so as to be taken as a chlorination reagent, thus having the advantages of safe and reliable operation and environmental protection.
Description
(1) Technical field The present invention relates to a synthetic method of an emtricitabine intermediate, in particular to (2R, 5S)-5-(5'-fluoro-cytosine-1-yl)-1,3-oxathiolane- Synthetic method of 2-carboxylate-L-menthyl ester. (2) Background technology Emtricitabine (Compound I) is an anti-AIDS drug developed in recent years. It is a new nucleoside reverse transcriptase inhibitor and is a highly efficient and selective inhibitor of HIV and HBV. There are many reports in the literature on the synthesis of emtricitabine, and the main routes are: (1) using glyoxylic acid as a raw material, reacting with 2,5-dithiane-1,4-diol, and then introducing chiral prosthetic groups, glycosides (Mansour T, et al EP: 515157 (1992)), (2) L-gulose obtained by multi-step reaction (Jeong L, et al. J Med Chem, 36 (2): 181— 195(1993)); (3) using (S)-(+)-mandelic acid as raw material, obtained by multi-step reaction (Keshava M., et al., US: 6380388, (2002)); (4) by Ethylene glycol is used as ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D411/04
Inventor 游金宗蒋善会
Owner JIANGSU COBEN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com