Process for production of intermediate for meropenem
A solid, compound technology, applied in the field of preparation of meropenem intermediates, can solve problems such as unsatisfactory implementation on an industrial scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
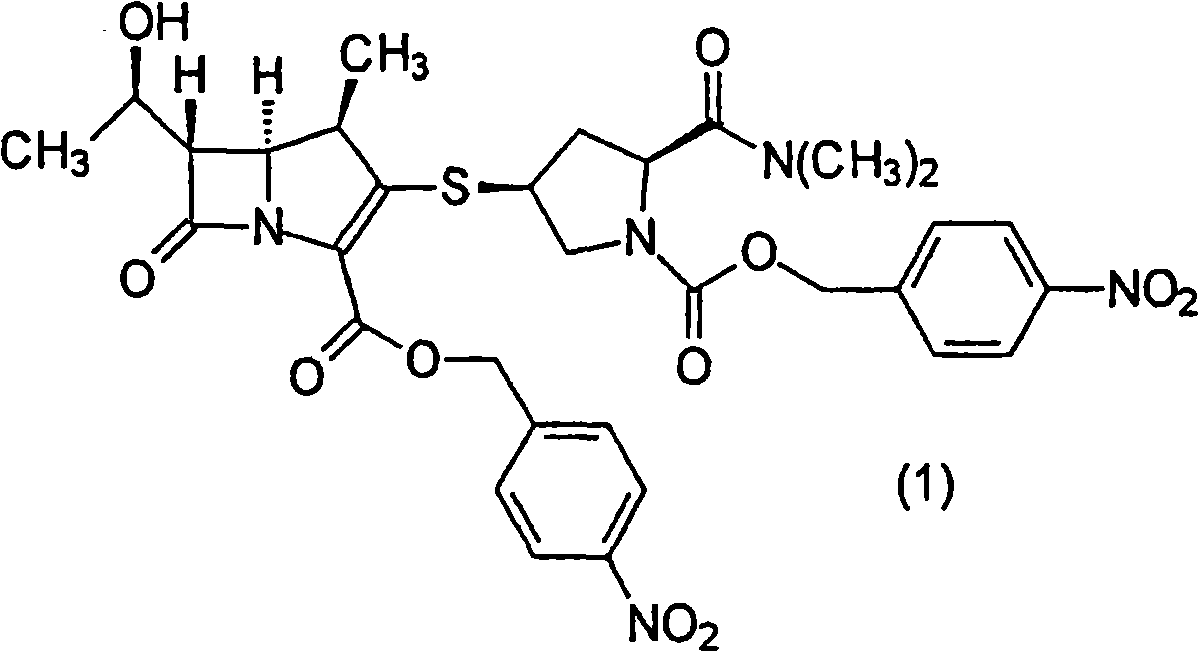
[0097] Preparation of (4R,5S,6S)-3-[[(3S,5S)-1-(p-Nitrobenzyloxycarbonyl)-5-(dimethylaminocarbonyl)-3-pyrrolidinyl]thio]- 6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate p-nitrobenzyl ester (1 ) in a good solvent solution
[0098] [chemical 5]
[0099]
[0100] 40.0g (4R, 5S, 6S)-3-diphenoxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo [3.2.0] p-nitrobenzyl hept-2-ene-2-carboxylate and 25.0g (2S, 4S)-2-dimethylaminocarbonyl-4-mercapto-1-(p-nitrobenzyloxycarbonyl )-pyrrolidine was added to 120ml of acetonitrile, and cooled to -10°C while stirring. 10.5 g of N,N-diisopropylethylamine was added over 10 minutes, followed by stirring at the same temperature for 3 hours. At 0-7°C, 240 ml of ethyl acetate and 200 ml of water were added to the reaction liquid, and stirred for 10 minutes. The aqueous layer was separated, and the obtained organic layer was washed twice with 200 ml of 10% brine and once with 200 ml of water a...
Embodiment 1
[0101] The separation of embodiment 1 compound (1)
[0102] Cool 50 ml of a water-saturated ethyl acetate solution of compound (1) obtained in Reference Example 1 to -7°C and stir. At this point, water precipitates out in the form of ice. 4 ml of n-hexane was added over 13 minutes, followed by stirring at -7°C to obtain a (water-saturated ethyl acetate / n-hexane) mixed solution of compound (1). After 4 ml of n-hexane was added in full, solids began to precipitate after 1 minute. Ten minutes after solid precipitation started, the temperature was raised to 10° C., followed by stirring for another 30 minutes. 45 ml of n-hexane was added over 2.5 hours, followed by stirring at the same temperature for 2 hours. The obtained slurry was filtered, and the obtained solid content was dried under reduced pressure to obtain a compound (1) as a solid (7.5 g of pure content).
[0103] The crystallinity of the obtained solid was 52% (the crystallinity was measured by the crystallinity mea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com