Trihydrate 3-amino propyl amine ethyl phosphorothioic acid high purity stable crystal and preparation thereof
A technology of aminopropylamine ethylthiophosphoric acid and trihydrate, which is applied in the direction of chemical instruments and methods, drug combinations, compounds of Group 5/15 elements of the periodic table, etc., and can solve the problems of purity, crystallization properties and stability. It can clarify other problems, achieve the effect of improving stability and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
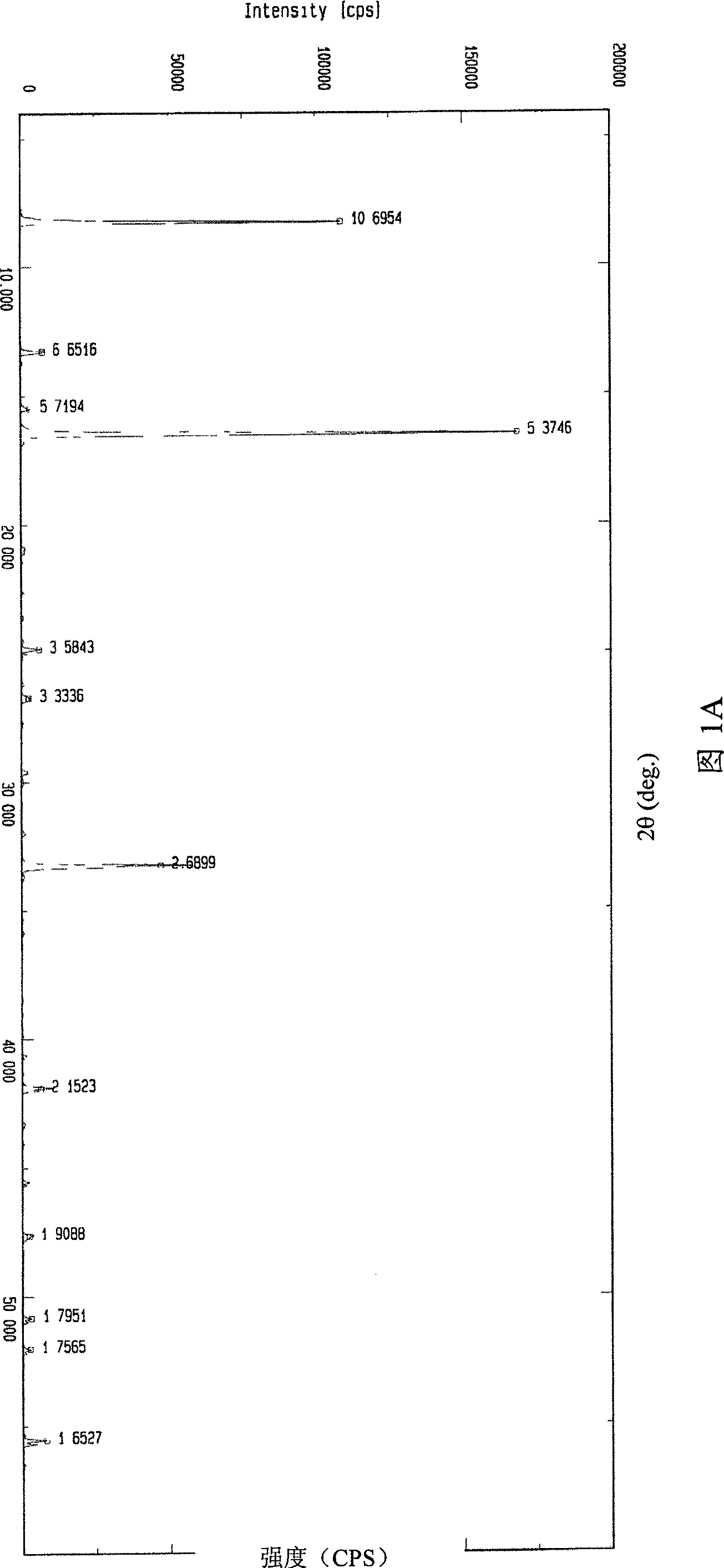
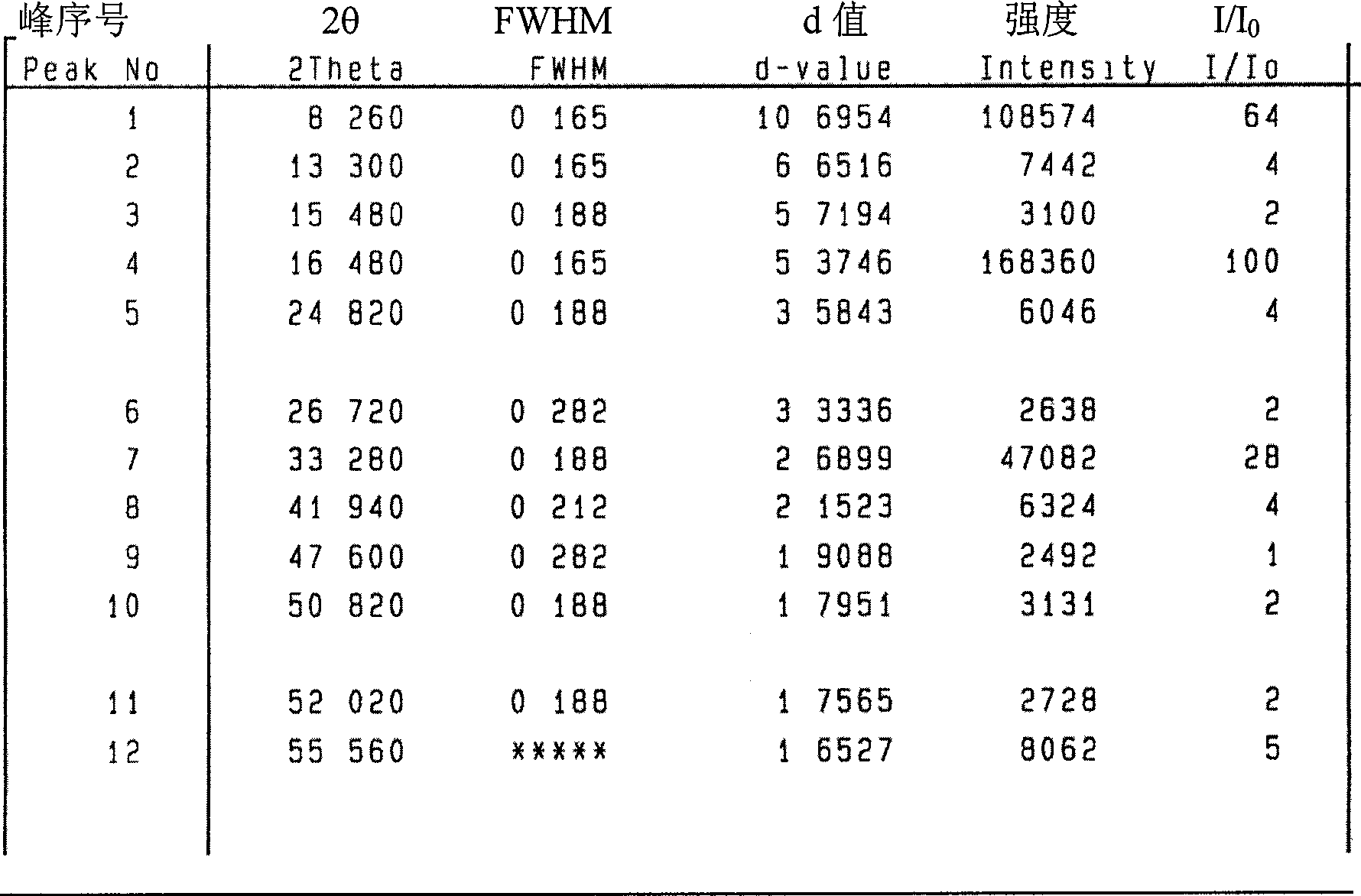
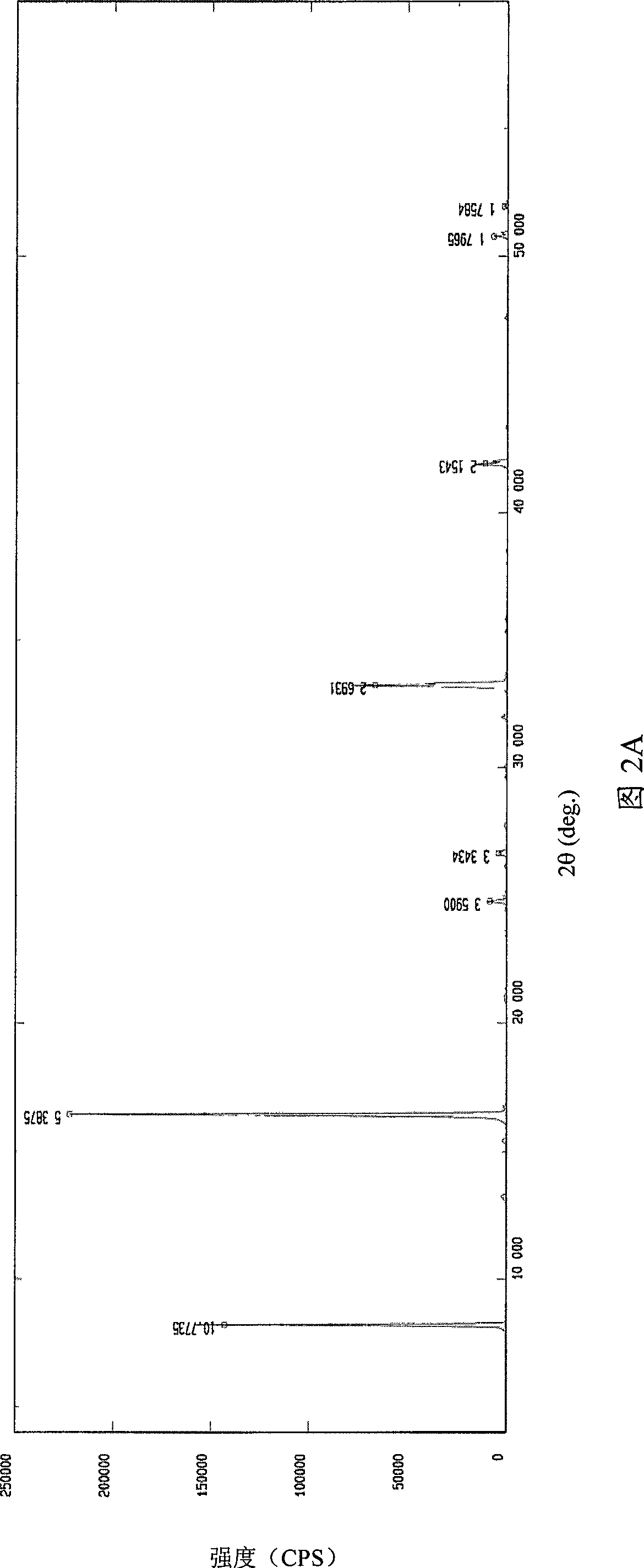
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of high-purity and stable 3-aminopropylamine ethyl phosphorothioate trihydrate
[0032] Step 1: Synthesis
[0033] Add 13.6kg of pure water and 19.2mol (7.6kg) of sodium thiophosphate dodecahydrate into a 50L stainless steel reactor, and add N-(2-bromoethyl)-1,3-propanediamine dihydrobromic acid under stirring. Salt 19.8mol (6.8kg, 3% excess), slowly add 11.0kg DMSO while maintaining the reaction temperature not exceeding 25°C. After dripping DMSO, monitor the reaction solution with silver nitrate solution until no black precipitate is precipitated. After the reaction is over, the reaction solution is cooled to 0° C., and stirred for 5 minutes every hour for 18 hours. After centrifugal filtration, 6.1 kg of crude 3-aminopropylamine ethyl phosphorothioate trihydrate was obtained.
[0034] Step 2: Purification by recrystallization for the first time
[0035] Add 22.0kg of pure water into a 50L stainless steel crystallization kettle, add 6.1kg of crude 3-...
Embodiment 2
[0040] Example 2 Preparation of high-purity and stable 3-aminopropylamine ethyl phosphorothioate trihydrate
[0041] Step 1: Synthesis
[0042] Add 13.6kg of pure water and 19.2mol (7.6kg) of sodium thiophosphate dodecahydrate into a 50L stainless steel reactor, and add N-(2-bromoethyl)-1,3-propanediamine dihydrobromic acid under stirring. Salt 19.5mol (6.7kg, 2% excess), stir to dissolve, slowly add 11.0kg DMSO while maintaining the reaction temperature not exceeding 25℃, monitor the reaction solution with silver nitrate solution, until no black precipitate precipitates, the reaction is over, the reaction solution is cooled down It was stirred at 0°C for 5 minutes per hour, maintained for 18 hours, and centrifuged to obtain 6.0 kg of 3-aminopropylamine ethyl phosphorothioate solid.
[0043] Step 2: Purification by recrystallization for the first time
[0044] Add 22.0kg of pure water to a 50L stainless steel crystallization kettle, add 6.0kg of synthetic crude 3-aminopropylamine ...
Embodiment 3
[0049] Example 3 Crystal purity of high-purity 3-aminopropylamine ethyl phosphorothioate trihydrate
[0050] Table 1 shows the comparison of the content of 3-aminopropylamine ethyl phosphorothioate trihydrate prepared by the method of the present invention and the content of amifostine on the market and the content of related substances:
[0051]
[0052] It can be seen from Table 1 that the content of amifostine trihydrate crystals prepared by the present invention is higher than that of amifostine trihydrate purchased on the market, and the related substances thiols, disulfide compounds and other related substances are much lower than the current ones. Products purchased on the market.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com