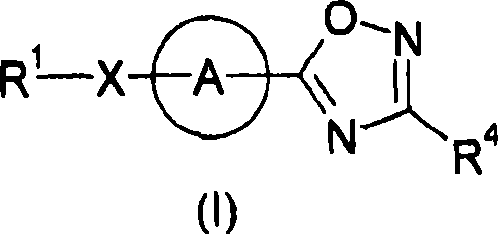
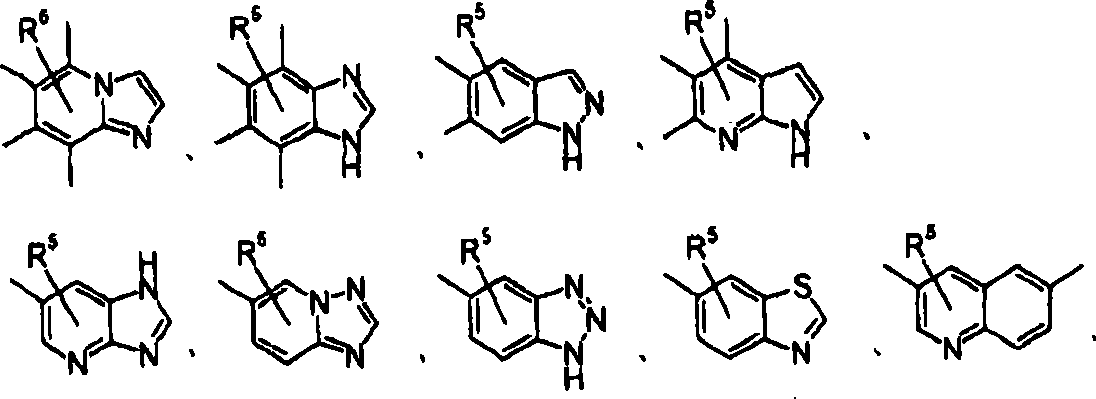
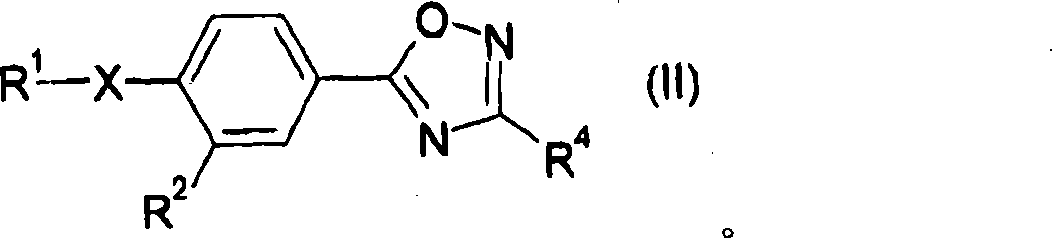
Hetero compound
A compound, heterocycloalkyl technology, applied in the field of pharmaceuticals, can solve problems such as useless observations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0176] Hereinafter, the present invention will be described in detail based on examples. The present invention is not limited to the invention of the compounds described in the following examples. In addition, the production method of the raw material compound is shown in the production example.
[0177] [In the following table, Pr represents the production example number, and "Structure" represents the structural formula. The abbreviation Me in the structural formula represents a methyl group, and Et represents an ethyl group. Crossed double bonds indicate a mixture of cis and trans forms, and when only numbers are written in the Data column, MS data are indicated. MS indicates mass analytical data. In the table, what is described as RT indicates the retention time in high performance liquid chromatography (HPLC), and M indicates minutes. The conditions of HPLC are expressed as: [column: Intertsil ODS-3 4.6×150mm, eluent 0.01M KH 2 PO 4 aq. / MeCN (3:7), flow rate: 1.0ml / ...
manufacture example 1
[0179] Imidazo[1,2-a]pyridine-7-carbonitrile hydrochloride (1.50g), hydroxylamine hydrochloride (301mg) and Na 2 CO 3 (3.50g) in CH 3 OH (57ml) was stirred at 60°C for 6 hours. The reaction liquid was cooled and concentrated, and the completion of the reaction was confirmed by LC-MS. Water was added to the residue, and extracted twice with EtOAc. The organic layer was washed with water and saturated brine, and washed with anhydrous MgSO 4 After drying and filtration, the filtrate was concentrated to give N'-hydroxyimidazo[1,2-a]pyridine-7-carboxamide (850 mg) as a white solid.
[0180] The compounds shown by Pr1-1 to Pr1-17 in the table were produced in the same manner as in Production Example 1.
[0181] [table 3]
[0182]
manufacture example 2
[0184] In a 50 ml reaction vessel, hydroxylamine (50% in water) was added to 1H-indole-4-carbonitrile (5.00 g) at room temperature in CH 3 OH (100ml) solution, refluxed for 15 hours (confirmed the completion of the reaction by TLC). The reaction solution was concentrated under reduced pressure and azeotroped with toluene three times. The resulting solid was washed with IPE. N'-Hydroxy-1H-indole-4-carboxyimidazolamide (6.12 g) was obtained in the form of a white solid.
[0185] The compounds shown in Pr2-1 to Pr2-26 in the table were produced in the same manner as in Production Example 2.
[0186] [Table 4]
[0187]
[0188] [table 5]
[0189]
[0190] [Table 6]
[0191] Pr NMR 2-16
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com