Methods of Handling Phenols
A fluid and reactor technology, applied in the continuous field of removing hydroxyacetone and methylbenzofuran, can solve the problem of low reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
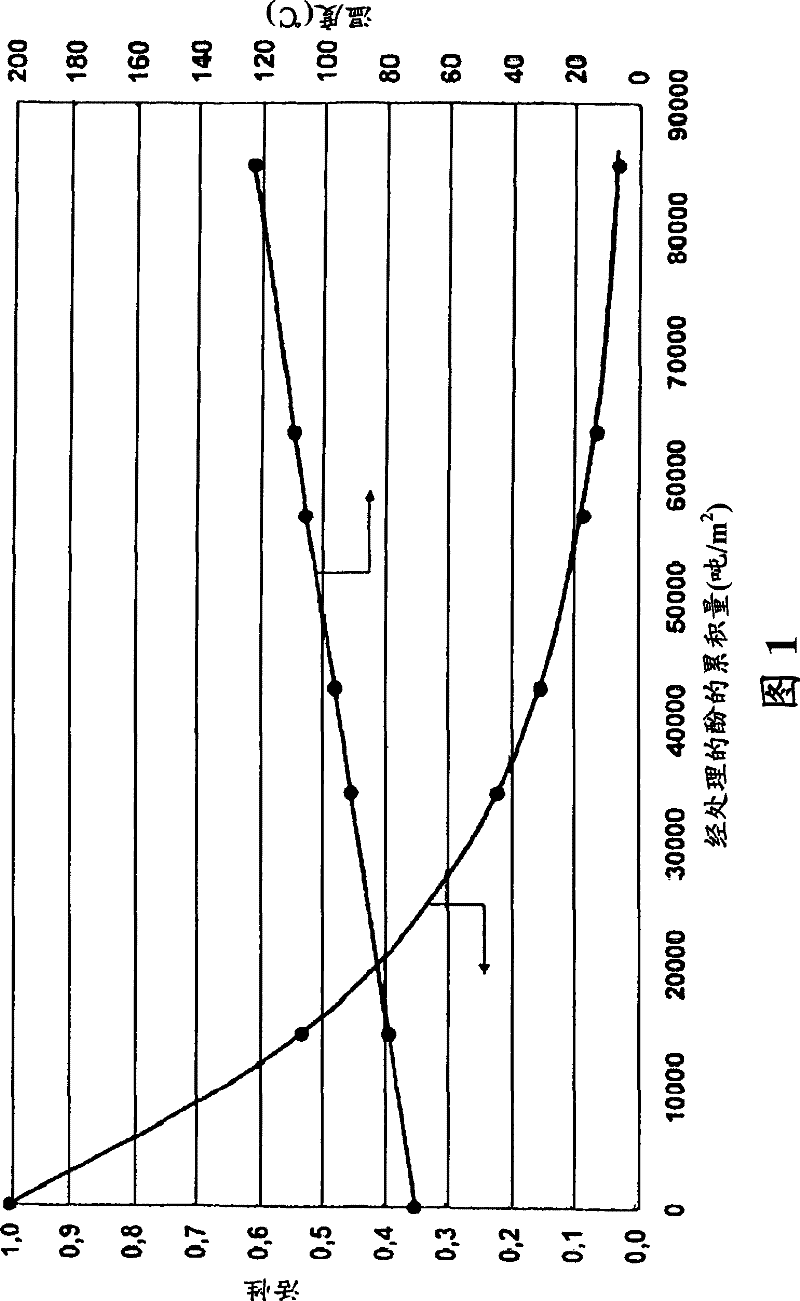
[0061] 1 kg / h of impure phenol stream containing 200 wppm hydroxyacetone was treated on ion exchange resin type K2431 from Lanxess. The plug flow reactor has a diameter of 0.025 m and a height of 1.2 m. The weight hourly space velocity WHSV (tonnes of phenol / hour / m3) in the plug flow reactor was thus 1.7. The temperature was adjusted so that the final concentration of hydroxyacetone after treatment was about 10 wppm. Fresh catalyst and catalyst samples from production units with varying degrees of usage were used. figure 1 The temperature required to compensate for the deactivation associated with the conversion of hydroxyacetone is shown. The activity of a new catalyst can be rated as "1.0". On this new ion exchange resin, a temperature of 70°C was sufficient to convert hydroxyacetone from 200 wppm to 10 wppm. The temperature must be increased to obtain the same conversion on the partially used ion exchange resin. For example, the temperature must be raised to 123°C to ...
Embodiment 2
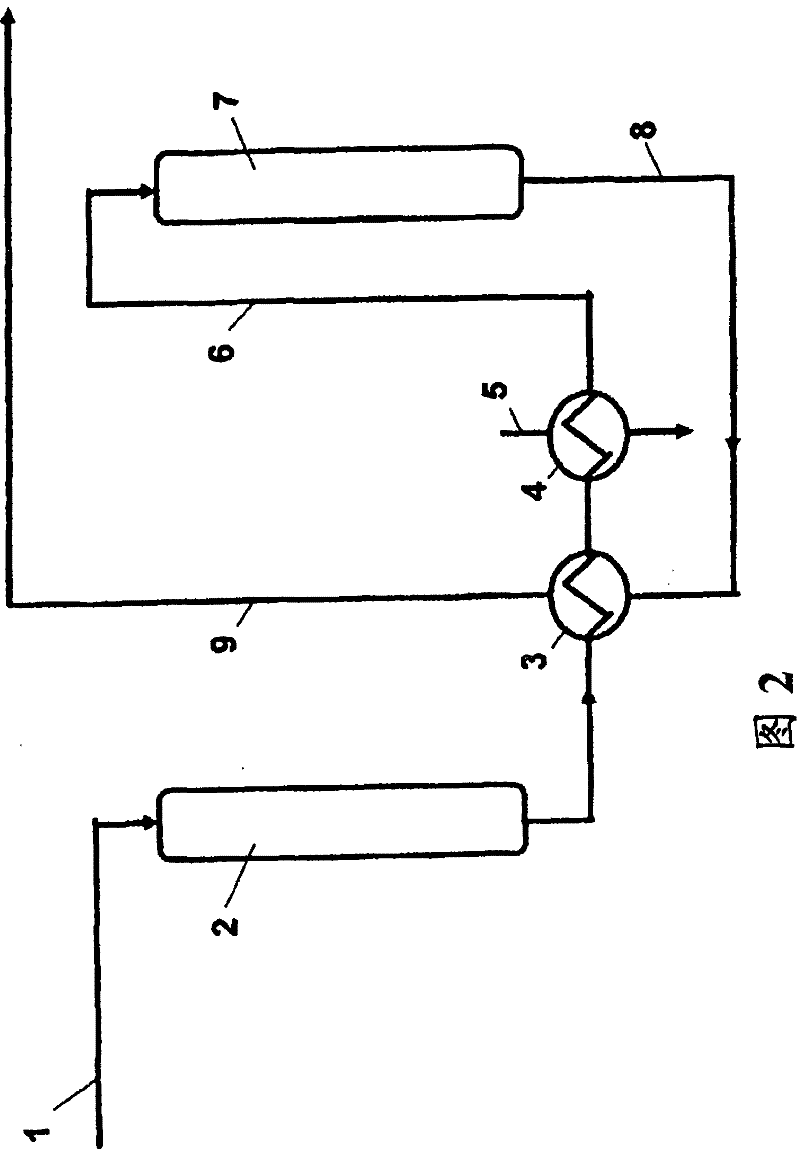
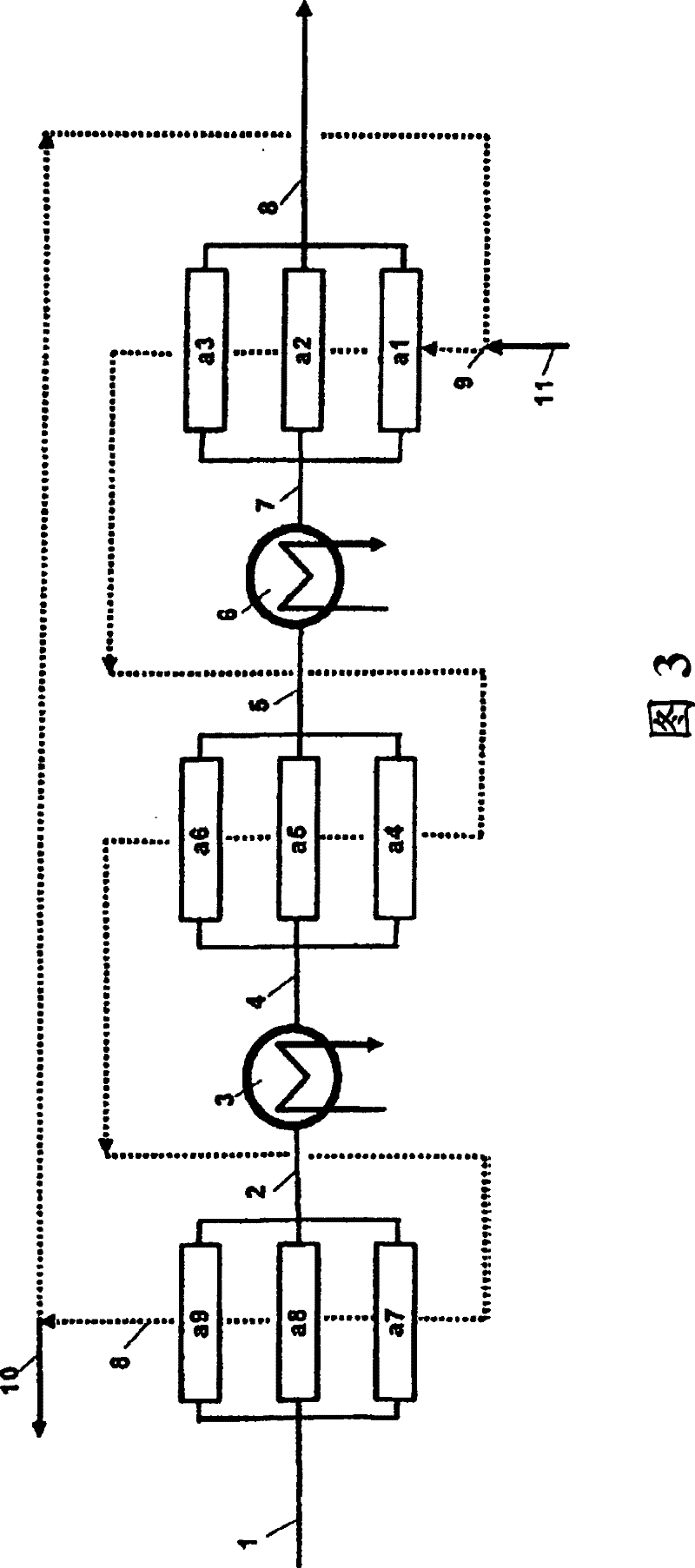
[0067] First, on the new fixed-bed ion exchange resin CT151 purchased from Purolite, the continuous treatment at 120°C was used figure 2 The impure phenol of comparative example 1 of the device has a weight hourly space velocity WHSV of 10. After the reactor, the phenol was cooled to 70°C and treated continuously at 70°C on a new fixed bed ion exchange resin CT151 from Purolite with a weight hourly space velocity WHSV of 2. The final concentration of hydroxyacetone was <1 wppm and the final concentration of methylbenzofuran was <10 wppm. Compared with Comparative Example 2, the same index of methylbenzofuran concentration <10 wppm was obtained with a volume less than half of the total reaction volume.
Embodiment 3
[0069] Similar to Example 2, firstly, the impure phenol of Comparative Example 1 was continuously treated at 120° C. on fixed-bed ion exchange resin CT151 purchased from Purolite. The activity is only 20% of the new ion exchange resin. The weight hourly space velocity WHSV is 2. The phenol was then cooled to 70°C and treated continuously at 70°C on a fresh fixed bed ion exchange resin with a weight hourly space velocity WHSV of 2. The final concentration of hydroxyacetone was <1 wppm and the final concentration of methylbenzofuran was 10 wppm. Compared with Comparative Example 2, only 70% of the total reaction volume is used to obtain the same index of methylbenzofuran concentration <10 wppm. Furthermore, again compared to Comparative Example 2, half of the total reactor volume was filled with low activity resin having an activity of only 20% of the new resin, so the ion exchange resin had a longer lifetime and thus was more economical.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com