Preparation method of butene oxidative dehydrogenation catalyst
A technology for oxidative dehydrogenation and catalysts, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve metal loss and zinc utilization rate is only 70-75% , can not be completely precipitated and other problems, to achieve the effect of maintaining the original activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
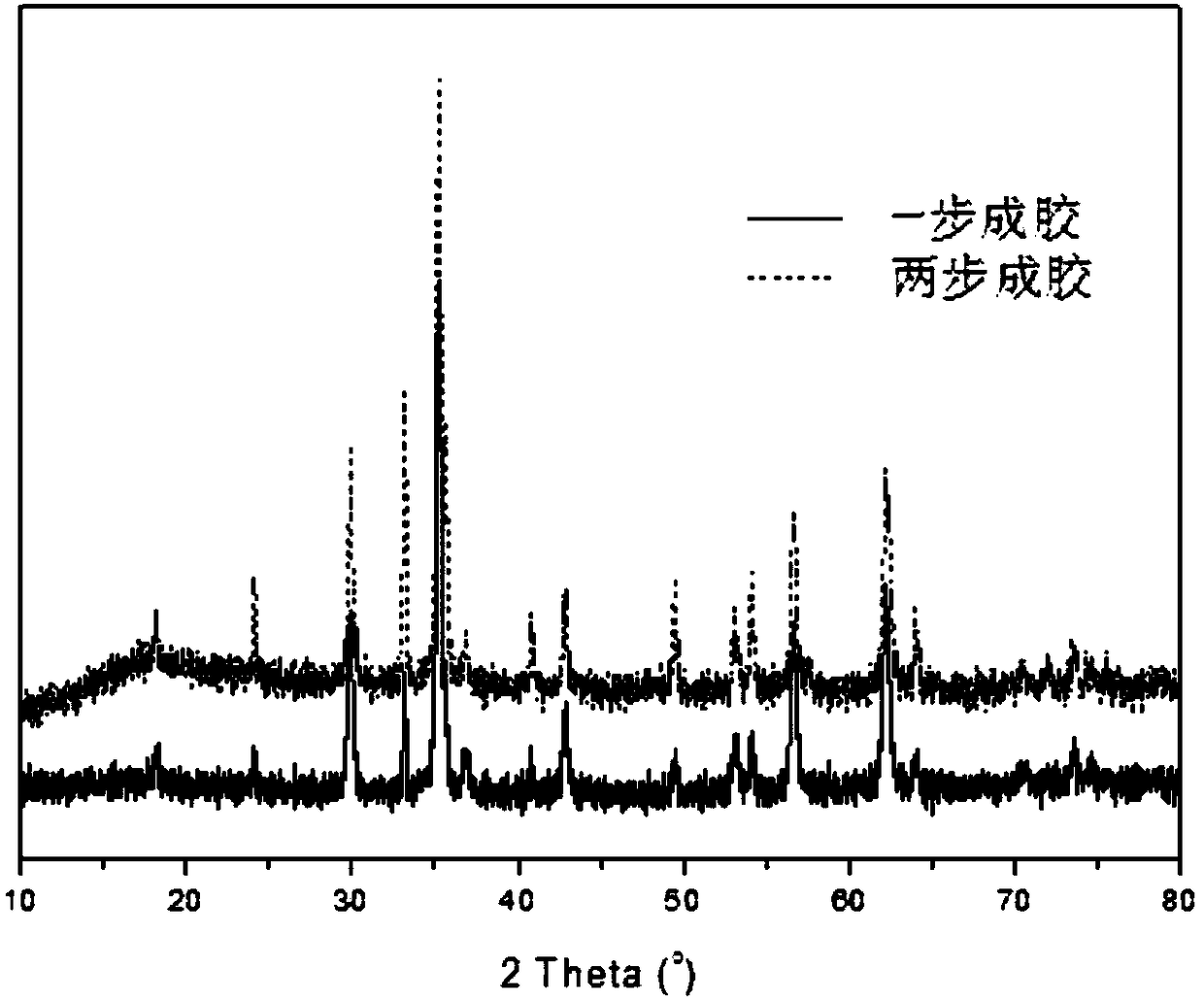
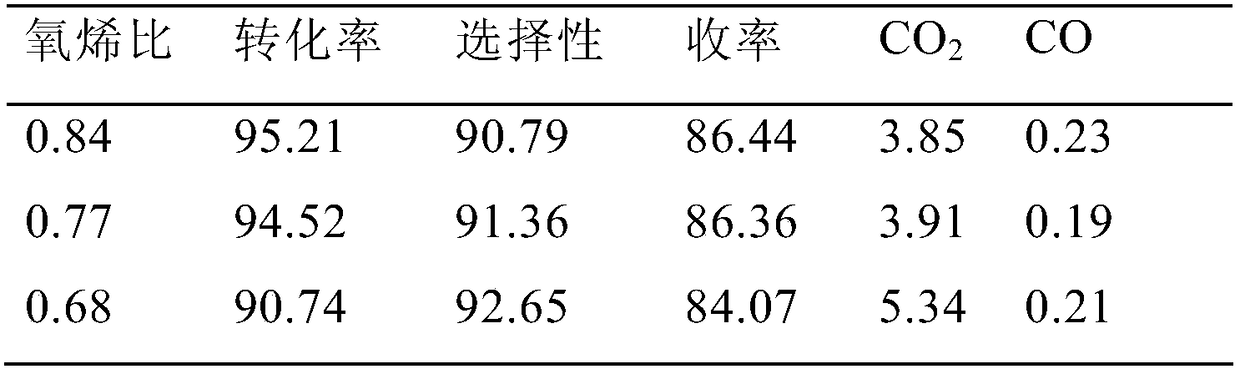
Image
Examples
Embodiment 1
[0028] Accurately weigh analytically pure Fe(NO 3 ) 3 9H 2 O 216.4g, analytically pure Zn(NO 3 ) 2 ·6H 2 O 71.2g, analytically pure Ca(NO 3 ) 2 ·6H 2 O 2.4g, add 1000ml deionized water to prepare a mixed solution. Gained solution is precipitated with 10% ammonia solution, and the pH value of the solution is monitored with a pH meter until the pH value is 7.6, the dropwise addition is stopped, and the first part of gelling is completed. At this time, carbon dioxide is passed into the solution at 600ml / min. Continued for 1 hour, stopped feeding carbon dioxide, then added 1.8g of scallop powder, after mixing well, the obtained precipitate was aged at 80°C for 1.0 hour. Wash 4 times with deionized water, 500ml of water for each wash. The loss of zinc in waste water is 12.6%, and the utilization rate of zinc in solid is 87.6%. It is dried at 120°C for 24 hours, placed in a muffle furnace at 700°C for 10 hours of roasting and activation, ground and sieved to take 20-60 mesh...
Embodiment 2
[0030] After 75g iron powder is dissolved completely through 540ml of 54% nitric acid, add analytically pure Zn (NO 3 ) 2 ·6H 2 O56.8g, analytically pure CaCl 2 0.8g, Ca(OH) 2 0.9g, add 1000ml deionized water to prepare a mixed solution. The resulting solution is precipitated with 10% ammonia solution, and the pH value of the solution is monitored with a pH meter until the pH value is 7.8, the dropwise addition is stopped, and the first part of gelling is completed. At this time, 400ml / min is passed into the solution for 1 hour, and the solution is stopped. Carbon dioxide was introduced, and 1.3 g of scallop powder was added. After mixing well, the obtained precipitate was aged at a constant temperature of 80° C. for 1.0 hour. Wash 4 times with deionized water, 500ml of water for each wash. The loss of zinc in wastewater is 9.45%, and the utilization rate of zinc in solids is 90.55%. After drying at 120°C for 24 hours, place it in a muffle furnace at 700°C for 10 hours of...
Embodiment 3
[0032] Accurately weigh analytically pure Fe(NO 3 ) 3 9H 2 O 536.9g, analytically pure Zn(NO 3 ) 2 ·6H 2 O125.6g, analytically pure CaCl 2 2g, Ca(OH)2 2g, add 2000ml deionized water to prepare a mixed solution. The resulting solution is precipitated with 10% ammonia solution, and the pH value of the solution is monitored with a pH meter, until the pH value is 7.9, the dropwise addition is stopped, and the first part of gelling is completed. At this time, 400ml / min is passed into the solution for 1 hour, and the solution is stopped. Carbon dioxide was passed through, and 3 g of scallop powder was added. After mixing well, the obtained precipitate was aged at a constant temperature of 85° C. for 1.0 hour. Wash 4 times with deionized water, 1000ml of water for each wash. The loss of zinc in waste water is 7.45%, and the utilization rate of zinc in solid is 92.55%. After drying at 120°C for 24 hours, put it in a muffle furnace for 700°C roasting and activation for 10 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com