Liranaftate cream preparation method
A technology of liranaphthate cream and liranaphthate, which is applied in the field of medicine, can solve the problems affecting the efficacy and stability of the medicine, and the particle size does not meet the regulations, and achieve the effect of small side effects, good stability and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
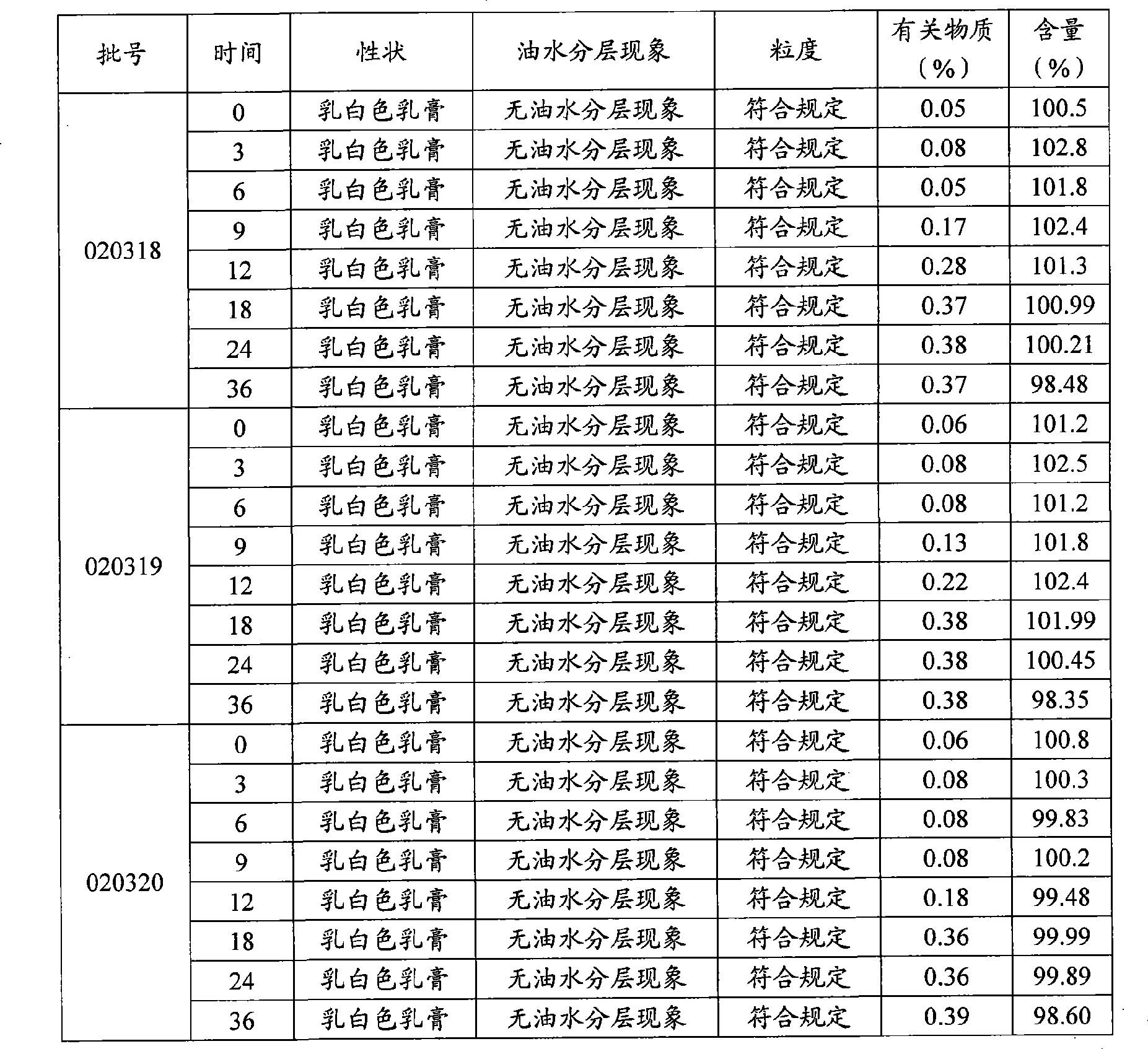
[0086] Embodiment 1 Preparation prescription (every 10000g)
[0087] Liranaftate 200g
[0088] Cetyl Alcohol 350g
[0089] Octadecanol 350g
[0090] Tween 80 500g
[0091] Glycerin 700g
[0092] Light liquid paraffin 800g
[0093] Glyceryl Stearate 400g
[0094] Methylparaben 10g
[0095] Ethylparaben 10g
[0096] 2,6-di-tert-butyl-p-cresol 20g
[0097] Preparation:
[0098] ① Airflow ultrafine grinding of liranaphtate, control particle size below 100 μm, set aside.
[0099] ②Preparation of oil phase: weigh cetyl alcohol, stearyl alcohol, light liquid paraffin, glyceryl stearate, methylparaben, ethylparaben, 2,6-di-tert-butyl p-paraffin Place cresol in an oil phase pot, heat to 70-80°C to melt into a uniform liquid, add liranaphthyl ester, stir at a low speed of 20-30 rpm to dissolve, keep warm at 70-80°C for later use.
[0100] ③Preparation of the water phase: Weigh the prescribed amount of Tween 80, glycerin and purified water into the water phase pot, heat to 70-...
Embodiment 2
[0102] Embodiment 2 preparation prescription (every 10000g)
[0103] Liranaftate 200g
[0104] Cetyl Alcohol 1600g
[0105] Octadecanol 1600g
[0106] Tween 80 800g
[0107] Glycerin 800g
[0108] Methylparaben 10g
[0109] 2,6-di-tert-butyl-p-cresol 20g
[0110] Purified water 4970g
[0111] Preparation:
[0112] ① Airflow ultrafine grinding of liranaphtate, control particle size below 100 μm, set aside.
[0113] ②Preparation of the oil phase: Weigh the prescribed amount of cetyl alcohol, stearyl alcohol, methylparaben, and 2,6-di-tert-butyl-p-cresol into the oil phase pot, heat to 70-80°C, Make it melt into a uniform liquid, add liranaphthyl ester, stir at a low speed of 20-30 rpm to dissolve, keep warm at 70-80°C for later use.
[0114] Other steps are the same as in Example 1 to obtain liranaftate emulsifiable cream of the present invention.
Embodiment 3
[0115] Embodiment 3 preparation prescription (every 10000g)
[0116] Liranaftate 200g
[0117] Cetyl Alcohol 800g
[0118] Octadecanol 800g
[0119] Tween 80 500g
[0120] Glycerin 1500g
[0121] Glyceryl Stearate 500g
[0122] Methylparaben 10g
[0123] 2,6-di-tert-butyl-p-cresol 20g
[0124] Purified water 6670g
[0125] Preparation:
[0126] ① Airflow ultrafine grinding of liranaphtate, control particle size below 100 μm, set aside.
[0127] ②Preparation of oil phase: Weigh cetyl alcohol, stearyl alcohol, glyceryl stearate, methyl paraben, 2,6-di-tert-butyl p-cresol in the oil phase pot and heat to 70-80°C to melt into a homogeneous liquid, add liranaphthate, stir at a low speed of 20-30 rpm to dissolve, and keep warm at 70-80°C for later use.
[0128] Other steps are the same as in Example 1 to obtain liranaftate emulsifiable cream of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com