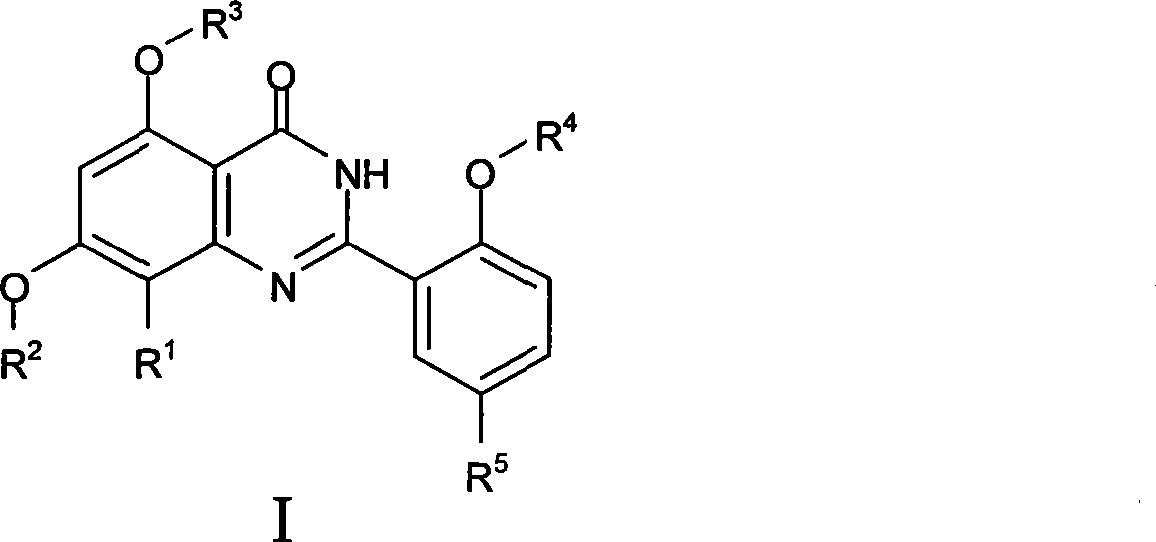
Quinazoline ketone derivant, preparation method and application thereof
A quinazolinone and compound technology, applied in the field of quinazolinone derivatives and their preparation, can solve the problems of photosensitivity, blurred vision, light-colored vision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1 2-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)-phenyl]-5,7-dimethoxy-8-bromo-quinazoline-4 (3H)-Kone
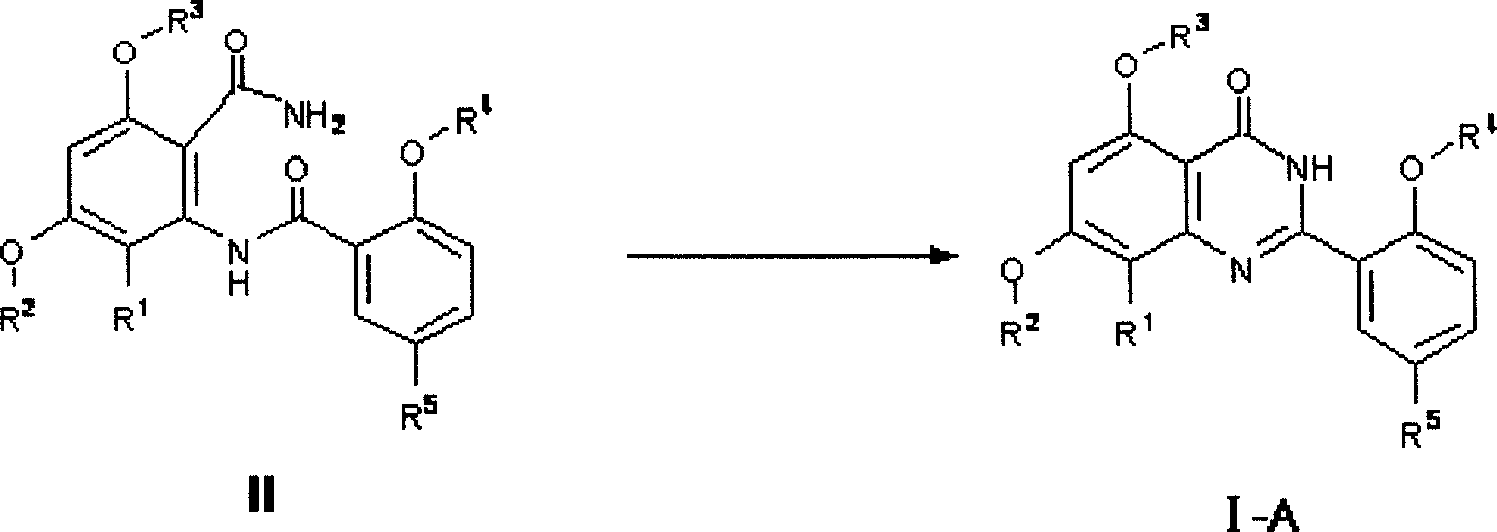
[0180] Step 1: 2-[2-Ethoxy-5-(4-methylpiperazine-1-sulfonyl)-benzamido]-5,7-dimethoxy-8-bromobenzamide
[0181]
[0182] Dissolve 2-ethoxy-5-[(4-methyl-1-piperazinyl)sulfonyl]benzoic acid (0.34g, 1mmol) in 20ml of dichloromethane, add carbonyldiimidazole (CDI, 3mmol) , stirred at room temperature for 0.5h, then added 2-amino-4,6-dimethoxy-3-bromobenzamide (0.28g, 1mmol) to the mixture, continued to stir for 1-6h, and analyzed with TLC Detection of reaction endpoints. After the reaction was completed, the mixed solution was washed with ammonium chloride solution and saturated brine, and the dichloromethane phase was dried with anhydrous magnesium sulfate, then concentrated to dryness under reduced pressure, and the residual solid was recrystallized with ethanol to obtain 0.51 g of a white powder with a yield of 86%. .
[0183] Step 2: Preparation of 2-{2...
Embodiment 2
[0186] Example 2 8-methyl-2-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)-phenyl]-5,7-dimethoxy-quinazoline- 4(3H)-keto
[0187]
[0188] Dissolve 408 mg (3.0 mmol) of anhydrous zinc chloride in 5 mL of dry NMP, add dropwise 2 mL of 2 mol / L methylmagnesium chloride (2.0 mmol) THF solution under ice cooling, and stir at room temperature for 1 hour to obtain methyl zinc reagent.
[0189] 2-[2-Ethoxy-5-(4-methylpiperazine-1-sulfonyl)-phenyl]-5,7-dimethoxy-8-bromo-quinazoline-4(3H) - Ketone 567mg (10i, 1.0mmol) was mixed in 5mL dry NMP, the methyl zinc reagent prepared above was added, and [(t-Bu)3P]2Pd 25mg (0.05mmol) was added under nitrogen protection, and heated at 40oC for 4 hours, Cool down to room temperature, add 10mL dichloromethane and 20mL water solution, extract the water phase once with 3mL dichloromethane, combine the organic phases, wash once with 2mL water and saturated brine, dry over anhydrous sodium sulfate, and perform silica gel column chromatography (CH2Cl...
Embodiment 3
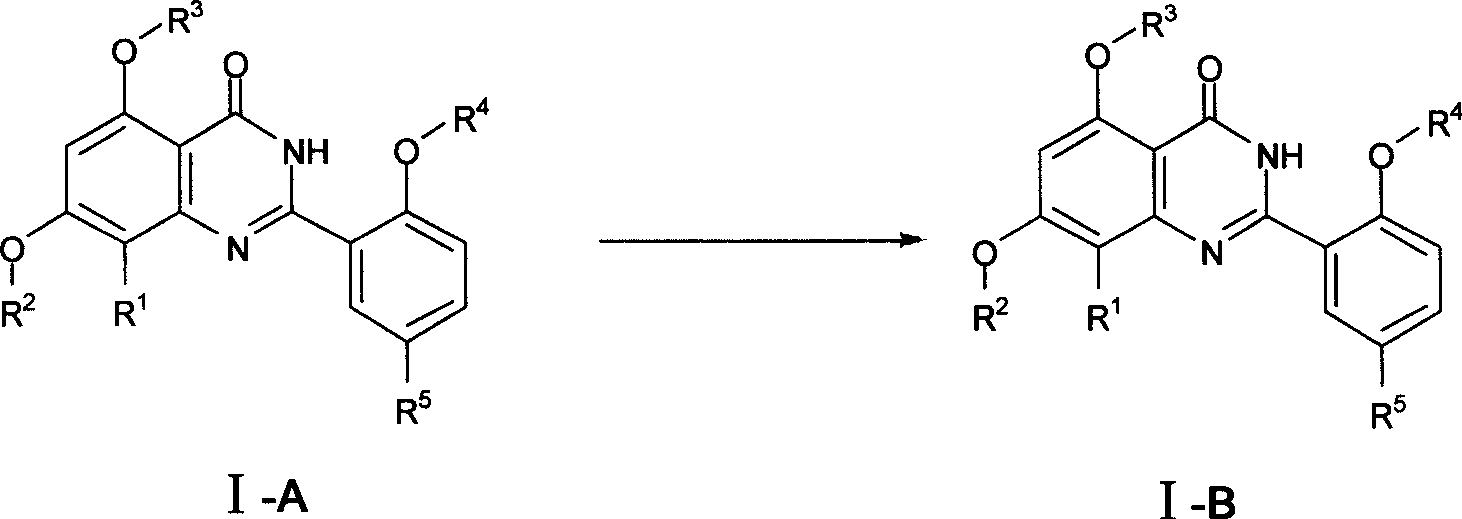
[0190] Example 3 2-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)-phenyl]-5-hydroxy-7-methoxy-8-bromo-quinazoline- 4(3H)-keto
[0191]
[0192] Dissolve the compound of Example 1 (0.57g, 1.0mmol) in 20mL of dry dichloromethane, add 2mL of boron tribromide (104uL, 1.1mmol) dichloromethane solution dropwise under cooling and stirring at -10oC, continue stirring for 4 hours, add 1mL diethyl ether, continue to stir for 30 minutes, add 20mL dichloromethane and 50mL 5% NaHCO3 to separate the liquid, extract the aqueous phase once with 10mL dichloromethane, combine the organic phases, wash once with 5mL 5% NaHCO3, saturated brine, anhydrous sulfuric acid Dry over sodium and recrystallize from petroleum ether / dichloromethane to obtain 0.45 g of light yellow solid with a yield of 82%. 1H-NMR (300MHz, CDCl3), δ 11.62(s, 1H), 10.82(brs, 1H), 9.11(d, J=2.3Hz, 1H), 7.90(dd, J=8.8, 2.3Hz, 1H), 7.17(d, J=8.8Hz, 1H), 6.57(s, 1H), 4.41(q, J=7.0Hz, 2H), 4.00(s, 3H), 3.18(m, 4H), 2.56(m, 4H )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com