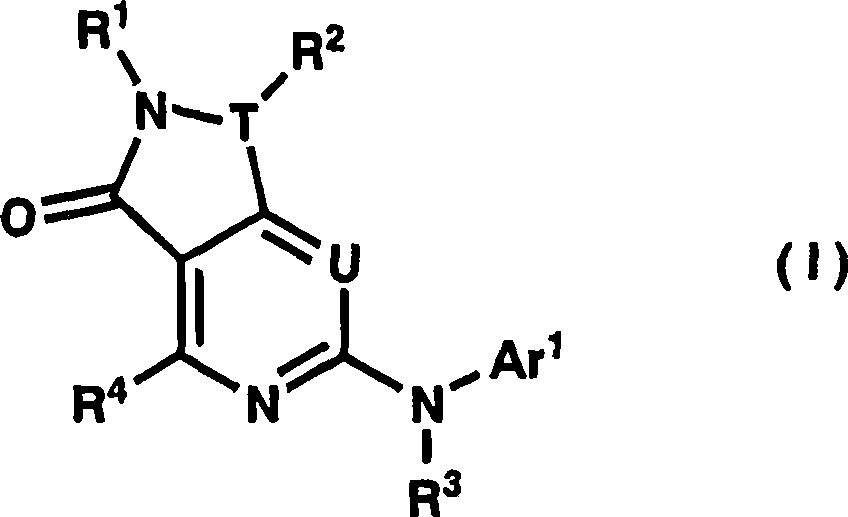
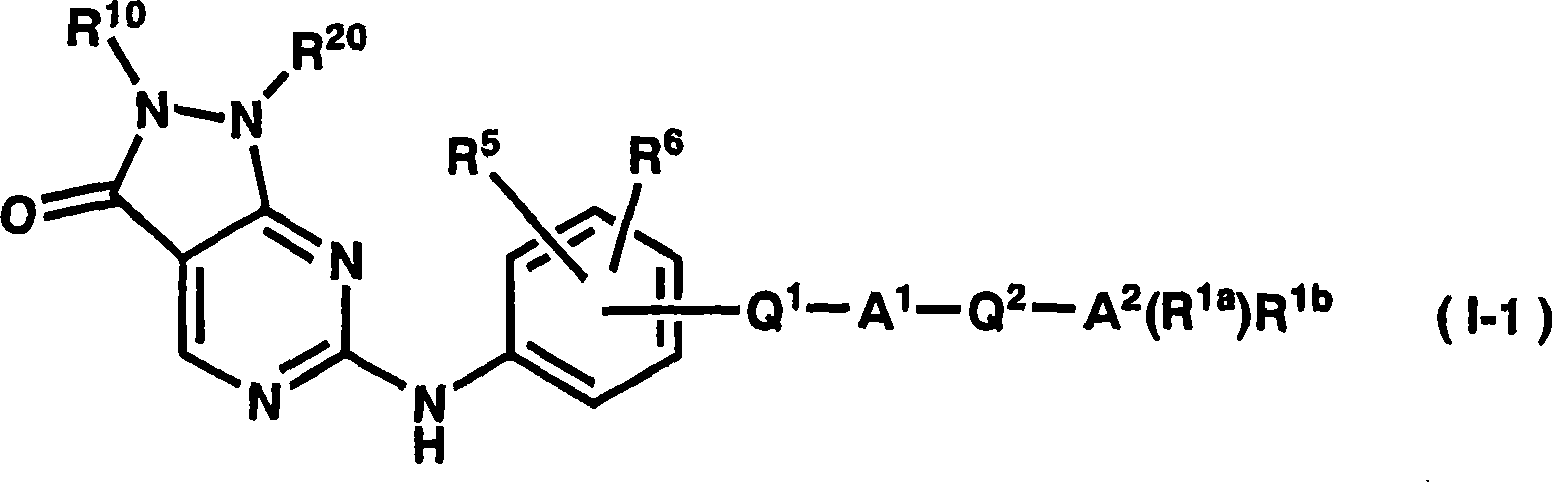
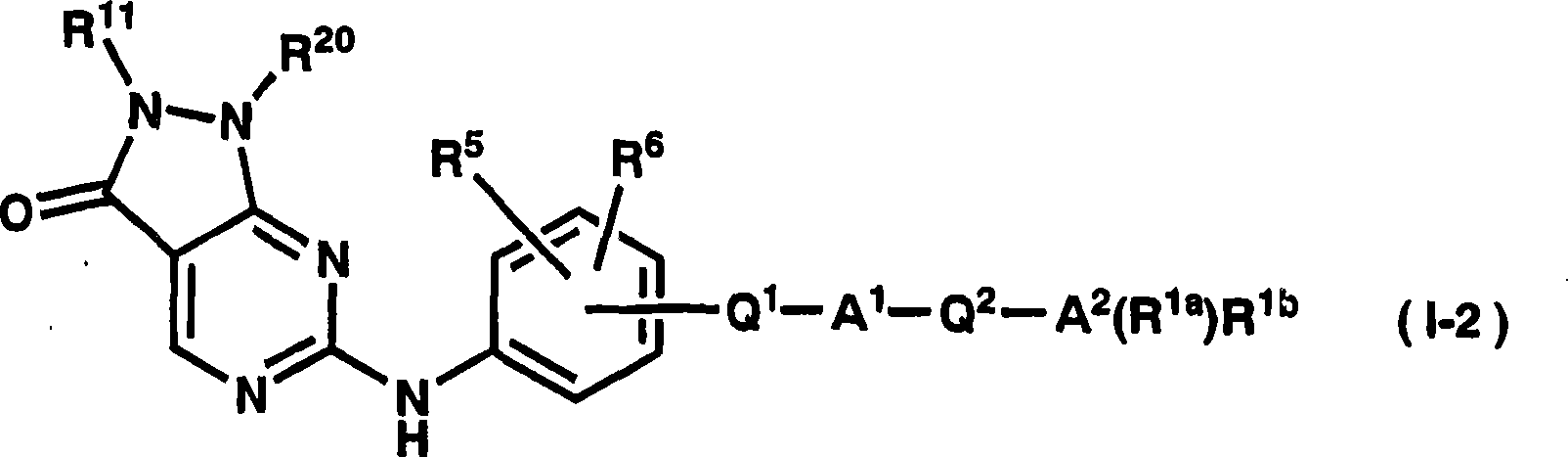
Dihydropyrazolopyrimidinone derivative
A compound, hydrogen atom technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0567] Preparation of 2-allyl-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
[0568] 1) 1-allylhydrazine carboxylic acid = tert-butyl ester
[0569] To a solution of 280 g of phthalic anhydride in 3 L of toluene was added 250 g of hydrazinecarboxylic acid = t-butyl ester. The reaction mixture was heated to reflux under a Dean-Stark trap for 3 hours. After cooling to room temperature, the resulting solid was collected by filtration to obtain 516 g of a crude product of (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)carbamate=tert-butyl ester.
[0570] 520 g of potassium carbonate, 43.3 g of benzyltriethylammonium chloride, and 250 mL of allyl bromide were sequentially added to a solution of the above compound in 3.5 L of acetonitrile, and stirred at room temperature for 18 hours. 1.5 L of water was added to the reaction solution, and the acetonitrile layer was separated and concentrated. 1 L of water was added to the residue and the aqueous layer, followed by extra...
preparation example 2
[0578] Preparation of 2-(2-chlorophenyl)-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
[0579] 1) Preparation of ethyl 4-[2-(2-chlorophenyl)hydrazino]-2-(methylthio)pyrimidine-5-carboxylate
[0580] To 4-chloro-2-(methylthio)pyrimidine-5-carboxylic acid ethyl ester 9.4g, 2-chlorophenylhydrazine hydrochloride 8.3g tetrahydrofuran 300mL solution was added N,N-diisopropylethyl Base amine 16.2mL, heated to reflux for 18 hours. After the solvent was concentrated under reduced pressure, water was added and extracted with ethyl acetate. The ethyl acetate layer was washed with saturated brine and dried over anhydrous sodium sulfate. After the solvent was distilled off under reduced pressure, ethyl 4-[2-(2-chlorophenyl)hydrazino]-2-(methylthio)pyrimidine-5-carboxylate was obtained as a crude yellow oil.
[0581] 2) Preparation of 2-(2-chlorophenyl)-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
[0582] To a solution of 13.8 g of the compound obtained...
preparation example 3
[0587] Preparation of 2-isopropyl-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
[0588] 1) Preparation of ethyl 4-hydrazino-2-(methylthio)pyrimidine-5-carboxylate
[0589] Add 15.0 g of ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate dissolved in 200 mL of ethanol to a solution obtained by dissolving 9.71 g of hydrazine monohydrate in 200 mL of ethanol and cooling to 0°C to obtain The solution was stirred for 1 hour. The precipitated solid was collected by filtration, washed with distilled water, and dried to obtain 9.66 g of the title compound as a white solid.
[0590] 1 H-NMR (400MHz, CD 3 OD)δ: 8.56(1H, s), 4.36(2H, q, J=7.2Hz), 2.62(3H, s), 1.39(3H, t, J=7.2Hz).
[0591] ESI-MS measured: m / z[M+H]+229
[0592] 2) Preparation of ethyl 4-[2-(1-methylethylene)hydrazino]-2-(methylthio)pyrimidine-5-carboxylate
[0593] 9.66 g of the above compound was dissolved in 300 mL of acetone, and stirred at 70° C. for 12 hours. After cooling the reaction so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com