Qualitative identification of Chinese medicine Bairui tablet and method for measuring kaempferol content therein
A technology of Bairui tablet and kaempferol, which is applied in pharmaceutical formulations, non-central analgesics, antipyretics and other directions, can solve problems such as influence on consumer interests, restriction of use, and failure to reflect the inherent quality of drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
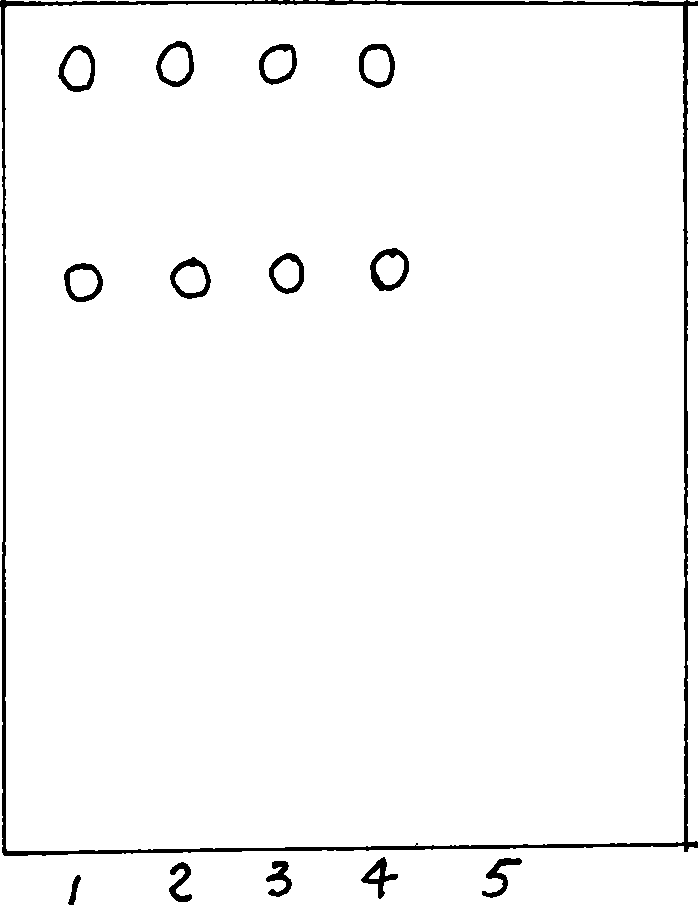
[0092] Example 1 Thin-layer identification of baishi sheet
[0093] Preparation of the test solution: take 5 pieces of each of the three batches (060201, 060202, 060203), remove the sugar coating, grind finely, add 20 ml of methanol, ultrasonically treat for 30 minutes, filter, evaporate the filtrate to dryness, add 2 ml of methanol to dissolve, as the test solution.
[0094] Preparation of blank sample solution: take 2 g of blank sample and prepare in the same way.
[0095] Preparation of the reference medicinal material solution: take 0.5 g of the reference medicinal material of balsam pear, add 10 ml of methanol, and prepare by the same method.
[0096] Thin-layer plate: silica gel G thin-layer plate; developing agent: n-butanol-acetic acid-water (4:1:5) upper layer solution; Spotting volume: control medicinal material solution, test solution, blank solution each 5μl, unfold, air Dry, viewed at 365 nm.
[0097] Result: In the chromatogram of the test substance, there are...
example 2
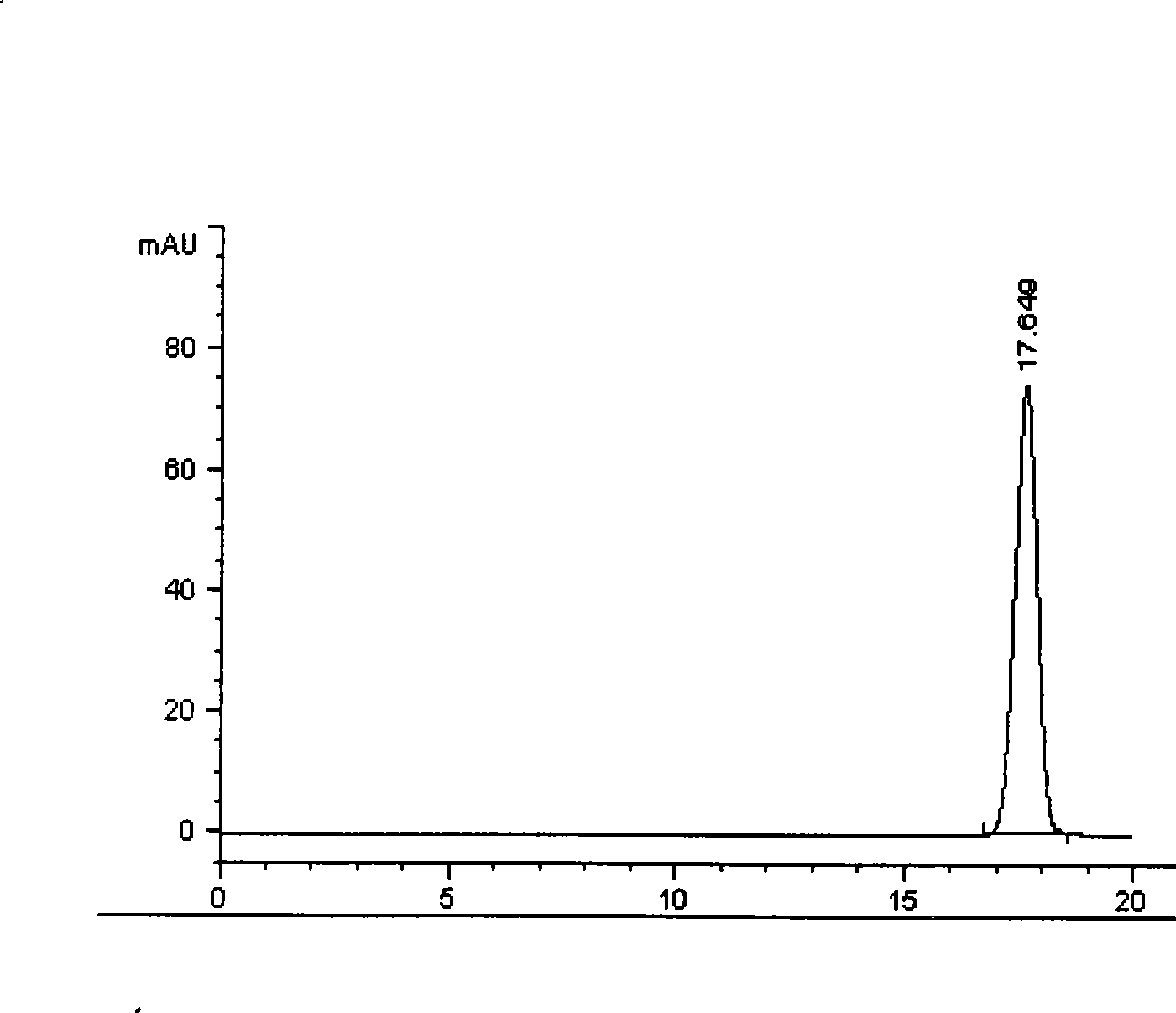
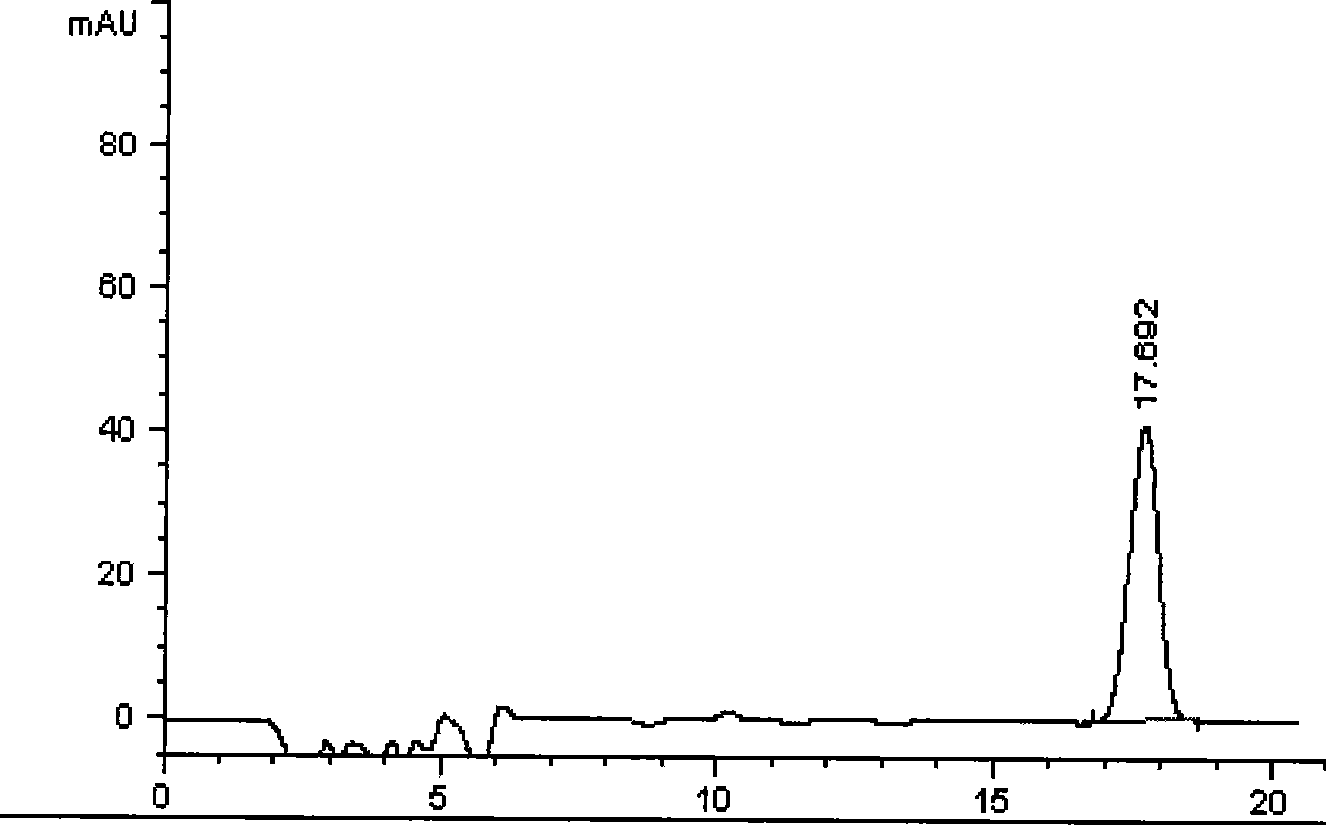
[0098] Example 2 Determination of kaempferol content in Bairui tablets
[0099] batch number Kaempferol (mg / tablet) 060201 0.61 060202 0.57 060203 0.58 average 0.59
[0100] (1) Preparation of reference substance solution: Take kaempferol reference substance, add methanol to make a solution containing 30 μg per 1ml, and use it as a reference substance solution.
[0101] (2) Preparation of the test solution: take 5 tablets of this product, remove the sugar coating, grind it finely, weigh 1 g, accurately weigh it, add 30 ml of 70% ethanol, 5 ml of 25% hydrochloric acid, and hydrolyze at 85°C-90°C for 60 minutes. Take out, cool rapidly, and dissolve to 50ml with 70% ethanol. Filtration, take the continued filtrate, that is.
[0102] (3) Chromatographic conditions and system suitability test: use octadecylsilane-bonded silica gel as filler, use methanol-0.5% phosphoric acid solution (50:50) V / V as mobile phase; flow rate is 1ml / min; detection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com