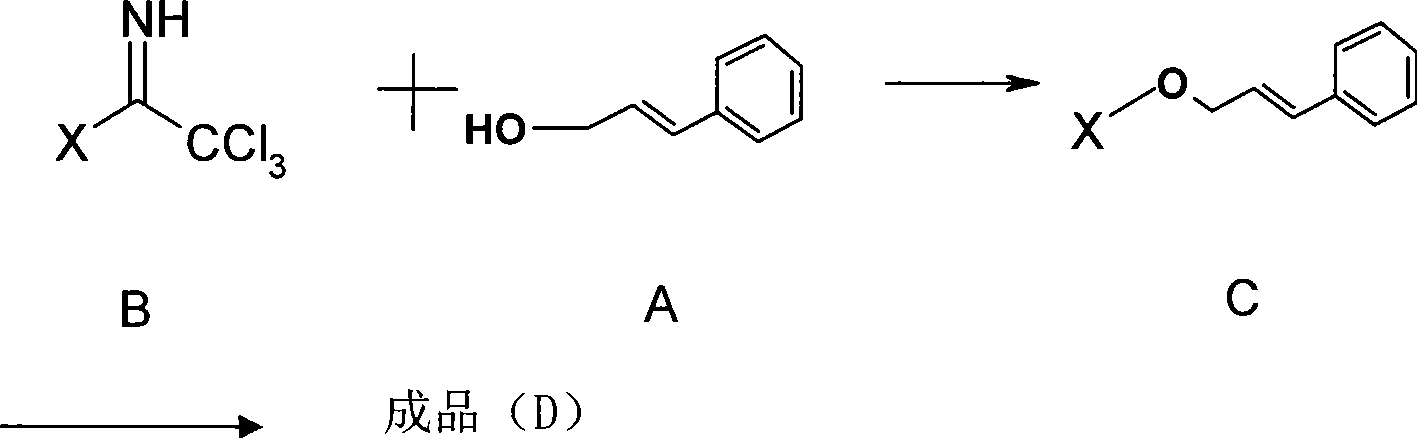
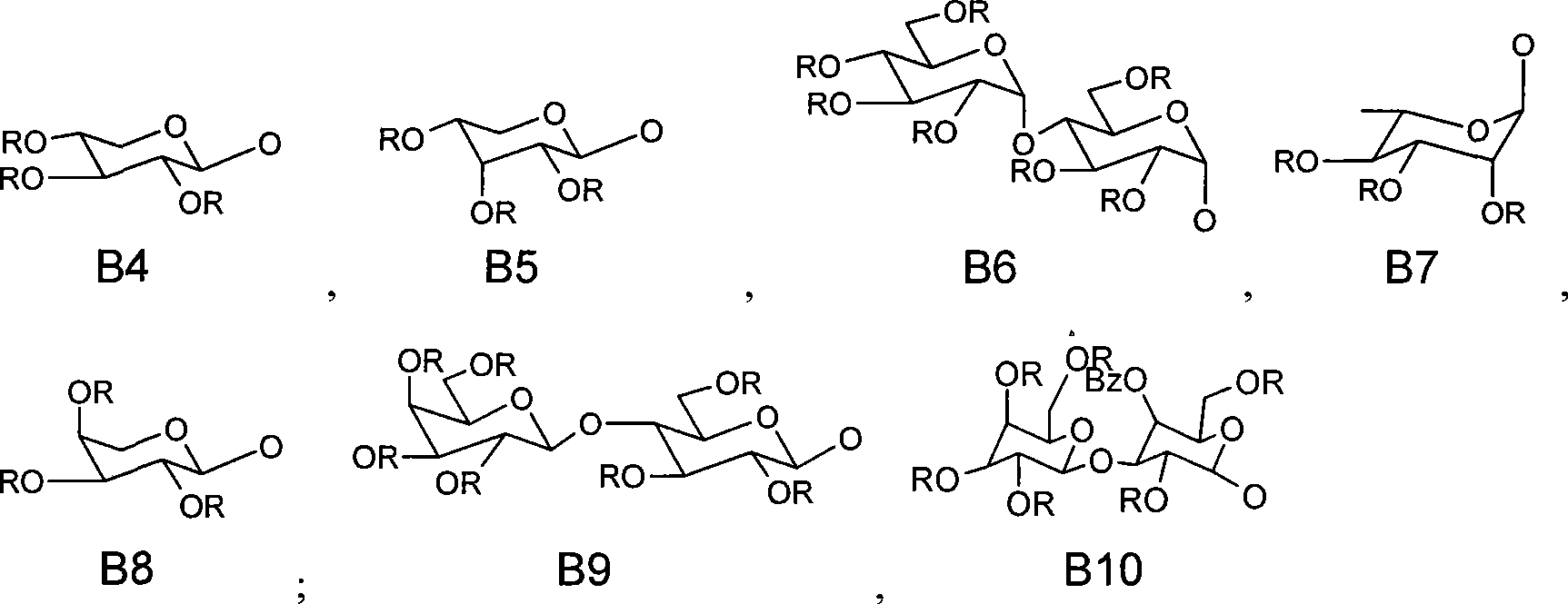
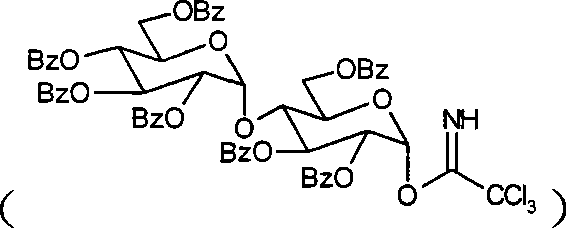
Method for preparing activity constituent rosavin derivates in rhodiola rosea and application
A synthesis method and reaction technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost, unsuitable for mass production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Glycosyl Donor Synthesis
[0019] 1) 2,3,4,5-Tetra-O-benzoylglucopyranoside Synthesis
[0020] At room temperature, add 170ml of dichloromethane into a 500ml three-neck round bottom flask with mechanical stirring, and start stirring. Glucose tetrabenzoate (34 g, 0.057 mol) was added and stirred until the solids were completely dissolved. Trichloroacetonitrile (10ml, 0.09mol) was added, and then DBU (1,8-diazabicyclo[5.5.4]-7-undecene), (0.68ml, 0.0046mol) was added by syringe. The reaction progress was monitored by TLC, and after the reaction was completed, the column was quickly purified.
[0021] 2) 2,3,4-tri-O-benzoyl-[6-O-(2',3',4'-tri-O-acetyl)-L-arabinofuranosyl]-D-pyridine glucopyranose trichloroacetimidate
[0022] (1) Synthesis of 6'-O-Tr-2', 3', 4'-tri-benzoyl-glucopyranosyl imidate
[0023] a) Synthesis of 6-O-triphenylmethyl-glucose
[0024] Dissolve D-glucose (180.16g, 1.0mol) in pyridine (1800ml), add TrCl (362g, 1.3mol) and DMAP (1.833...
example 2
[0060] Synthesis of Example 2 Fully Protected Rosavins
[0061] 1) Fully protected Rosin Synthesis
[0062] Add cinnamyl alcohol, perbenzoylated glucosiminate, and 4A molecular sieves into an eggplant-shaped bottle, vacuumize, and fill with argon; the above operation is repeated once. Add anhydrous dichloromethane, stir at room temperature for 30 minutes, then cool in an ice-salt bath, add TMSOTf / dichloromethane solution dropwise in batches, react at low temperature for 20 minutes, rise to room temperature and quench with triethylamine. The reaction process was monitored by TLC, the reaction solution was filtered, the filter residue was washed with dichloromethane, the filtrate was concentrated to dryness under reduced pressure, separated by silica gel column chromatography, the eluent containing the product was concentrated to dryness under reduced pressure, and dried by a vacuum pump to obtain a light yellow foam solid product. The yield is 50-80%
[0063] (2) Fully p...
example 3
[0082] Example 3 Synthesis of Rosavins
[0083] 1) Rosin Synthesis
[0084] Add the full-protection product, dichloromethane, and stir to dissolve in an eggplant-shaped bottle, then add methanol, and then add a catalytic amount of sodium methoxide, and stir overnight at room temperature. TLC monitors the reaction process, and the reaction solution is concentrated to dryness under reduced pressure, separated by silica gel column chromatography, and purified by column chromatography (CHCl 3 / MeOH) to obtain highly pure white solid rosin.
[0085] (2) Rosarin Synthesis
[0086] Add the full-protection product, dichloromethane, and stir to dissolve in an eggplant-shaped bottle, then add methanol, and then add a catalytic amount of sodium methoxide, and stir overnight at room temperature. TLC monitors the reaction process, and the reaction solution is concentrated to dryness under reduced pressure, separated by silica gel column chromatography, and purified by column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com