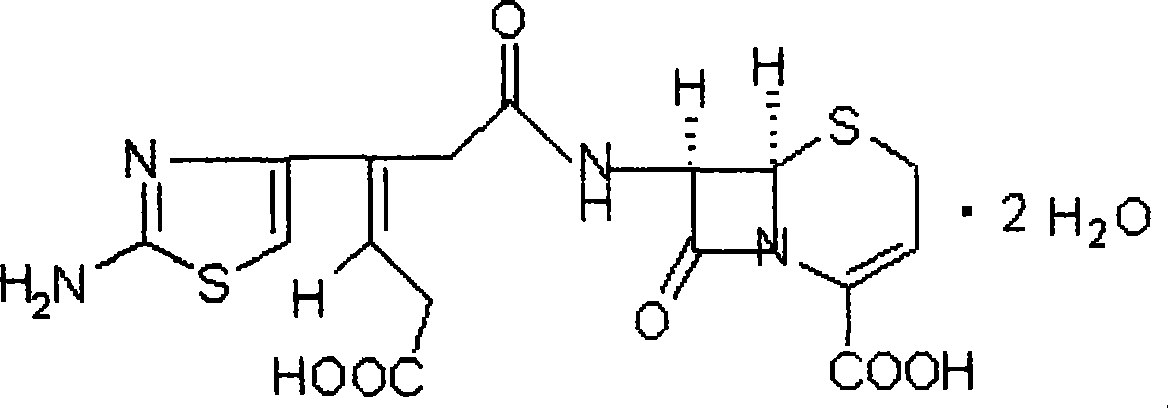
Ceftibuten suspension
A ceftibuten and suspension technology, applied in the direction of emulsion delivery, medical preparations of non-active ingredients, organic active ingredients, etc., can solve problems such as inconvenience, and achieve high quality, good stability, and simple methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: specification 5ml: 0.2g (1000)
[0030] prescription:
[0031] Ceftibuten 200g
[0032] Vitamin C 10g
[0033] Syrup (15%) 4600g
[0034] Carboxymethylcellulose 50g
[0035] Ethylparaben 2g
[0036] Agar 20g
[0037] Flavor 1g
[0038] Preparation method:
[0039] Swell the prescribed amount of carboxymethyl cellulose and agar in 200ml of 85°C hot water, set aside, measure the prescribed amount of vitamin C, syrup, and essence and mix evenly, add the above solution, and mix well. Add ceftibuten and stir well to disperse, then dissolve ethylparaben in 10ml ethanol and add to the mixed solution, stir, mix evenly, measure the content of intermediates, subpackage, inspect and pack the finished product.
Embodiment 2
[0040] Embodiment 2: specification 5ml: 0.2g (10000)
[0041] prescription:
[0042] Ceftibuten 2000g
[0043] Vitamin C 100g
[0044] Syrup (15%) 46000g
[0045] Carboxymethylcellulose 500g
[0046] Ethylparaben 20g
[0047] Agar 200g
[0048] Flavor 10g
[0049] Preparation method:
[0050] Swell the prescribed amount of carboxymethyl cellulose and agar in 2000ml of 85°C hot water, set aside, measure the prescribed amount of vitamin C, syrup, and essence and mix evenly, add the above solution, and mix well. Add ceftibuten and stir well to disperse, then dissolve ethylparaben in 100ml ethanol and add to the mixed solution, stir, mix evenly, measure the content of intermediates, sub-package, finished product inspection, and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com