Bisoprolol fumarate dispersible tablet and preparation method thereof
A technology of bisoprolol fumarate and dispersible tablets, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
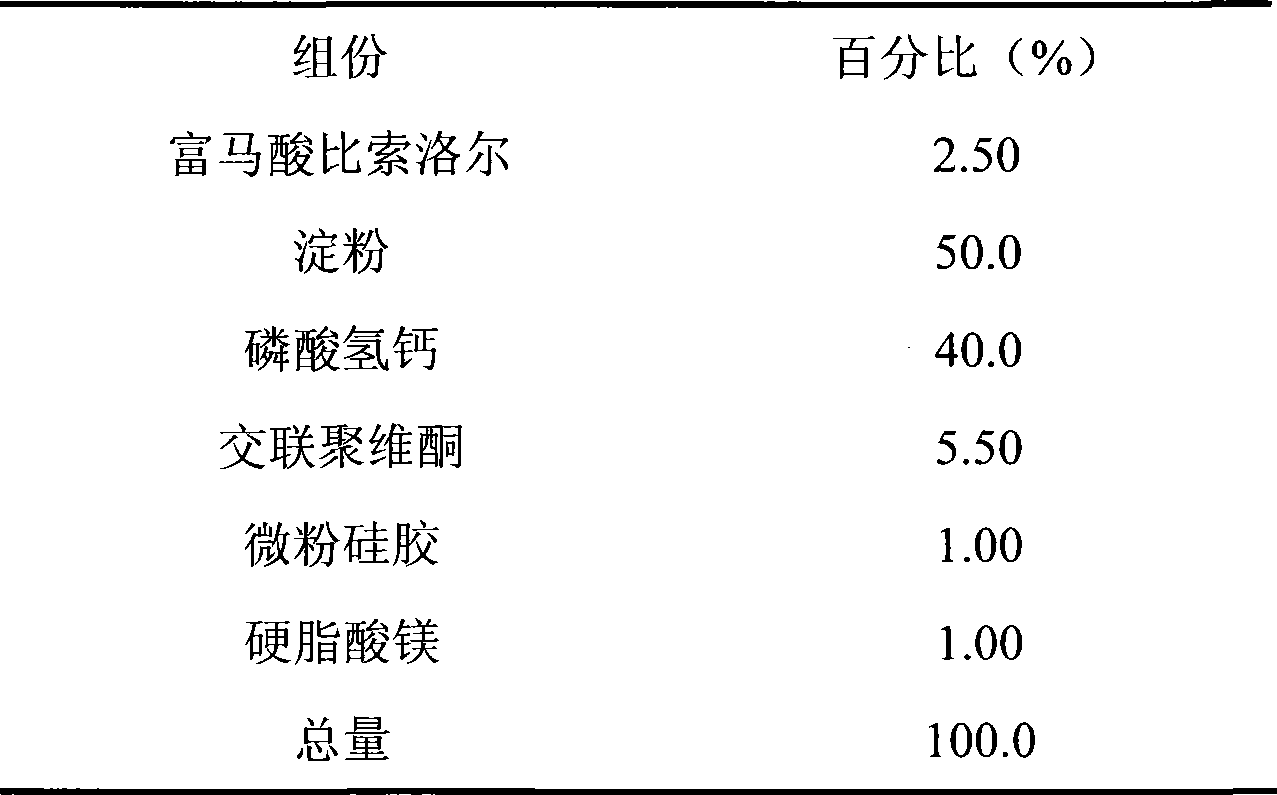
Embodiment 1
[0024]
[0025] Preparation:
[0026] Weigh half of the prescription amount of microcrystalline cellulose PH101 and crospovidone and the prescription amount of calcium hydrogen phosphate; after mixing, make soft material with water, granulate with 18 mesh sieve, bake in an oven at 50°C for 2 hours, take out Use a 30-mesh sieve to sieve the granules, mix the obtained granules with the prescribed amount of bisoprolol fumarate, micropowder silica gel, magnesium stearate, and the remaining microcrystalline cellulose PH101 and crospovidone, and then use a 6mm dimple Punching tablets are available.
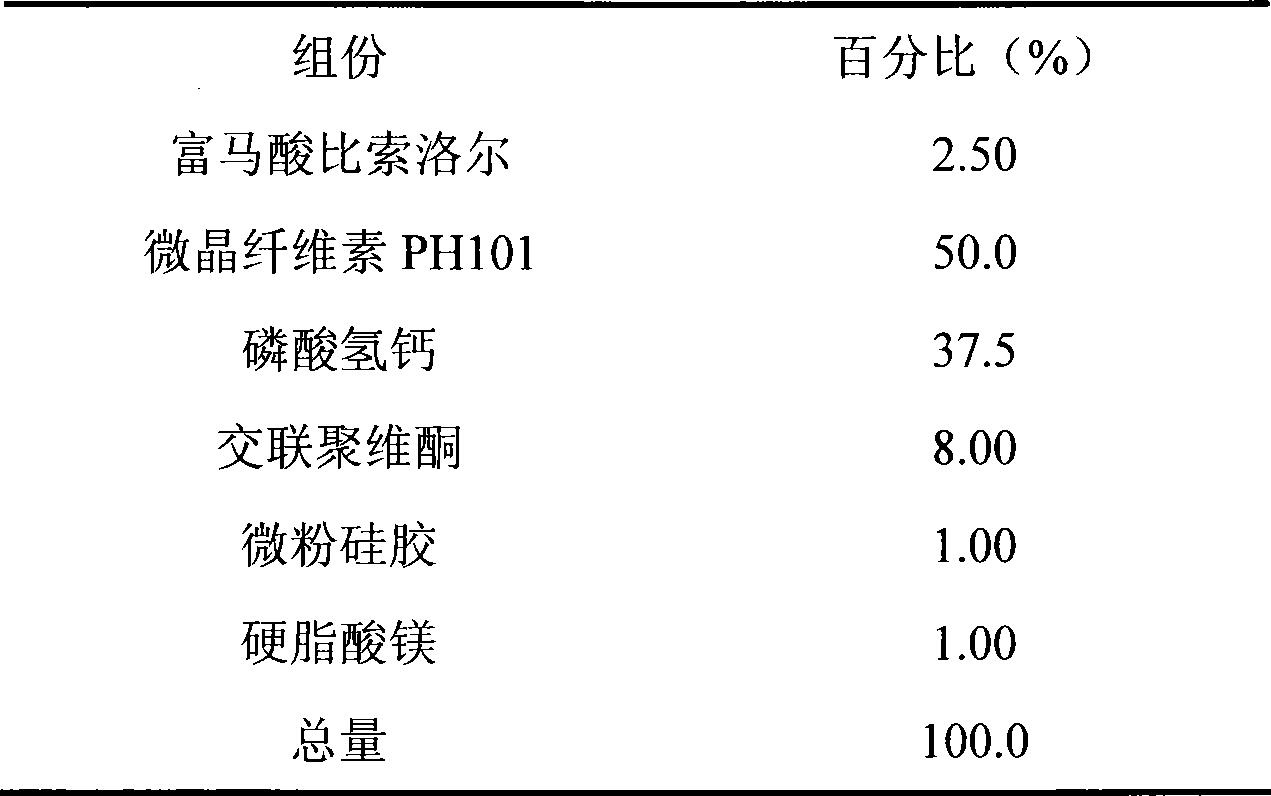
Embodiment 2
[0028]
[0029] Preparation:
[0030] Weigh half of the prescription amount of microcrystalline cellulose PH101 and crospovidone and the prescription amount of calcium hydrogen phosphate; after mixing, make soft material with water, granulate with 18 mesh sieve, bake in an oven at 50°C for 2 hours, take out Use a 30-mesh sieve to sieve the granules, mix the obtained granules with the prescribed amount of bisoprolol fumarate, micropowder silica gel, magnesium stearate, and the remaining microcrystalline cellulose PH101 and crospovidone, and then use a 6mm dimple Punching tablets are available.
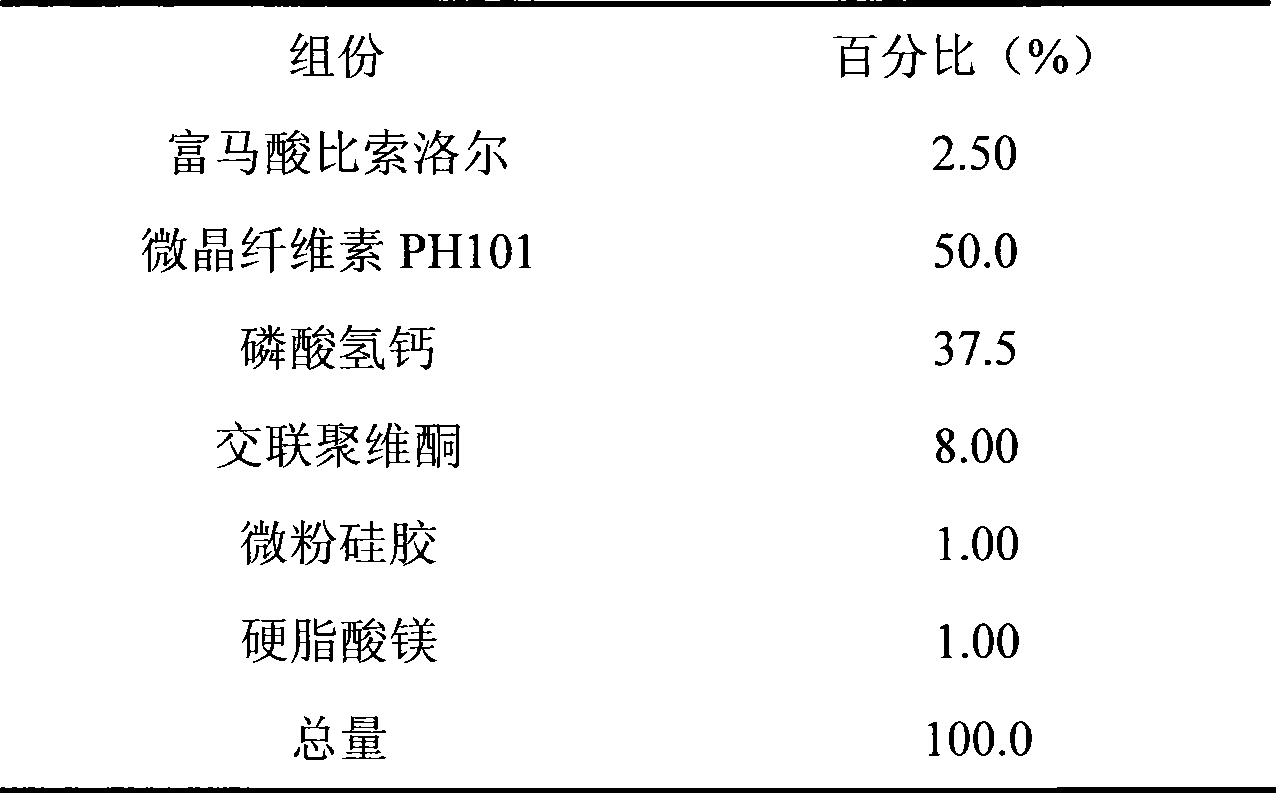
Embodiment 3
[0032]
[0033] Preparation:
[0034] Weigh half of the prescription amount of microcrystalline cellulose PH101 and crospovidone and the prescription amount of calcium hydrogen phosphate; after mixing, make soft material with water, granulate with 18 mesh sieve, bake in an oven at 50°C for 2 hours, take out Use a 30-mesh sieve to sieve the granules, mix the obtained granules with the prescribed amount of bisoprolol fumarate, micropowder silica gel, magnesium stearate, and the remaining microcrystalline cellulose PH101 and crospovidone, and then use a 6mm dimple Punching tablets are available.
[0035] Comparative example 1 compares with the dissolution rate of embodiment 1, 2, 3 and commercially available tablet at 5 minutes:
[0036] Measure the dissolution rate in 5 minutes by the 2005 edition Chinese Pharmacopoeia appendix XC dissolution test method, the results are as follows:
[0037]
[0038] From the results, it can be seen that the dissolution rate of the pharm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com