Recombinant lactobacillus and use of the same
A technology of Lactobacillus and Lactobacillus casei, which is applied in the direction of bacteria, drug combinations, and medical preparations containing active ingredients, etc., can solve the problems of unsatisfactory treatment of mite allergy and impossibility of disease prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
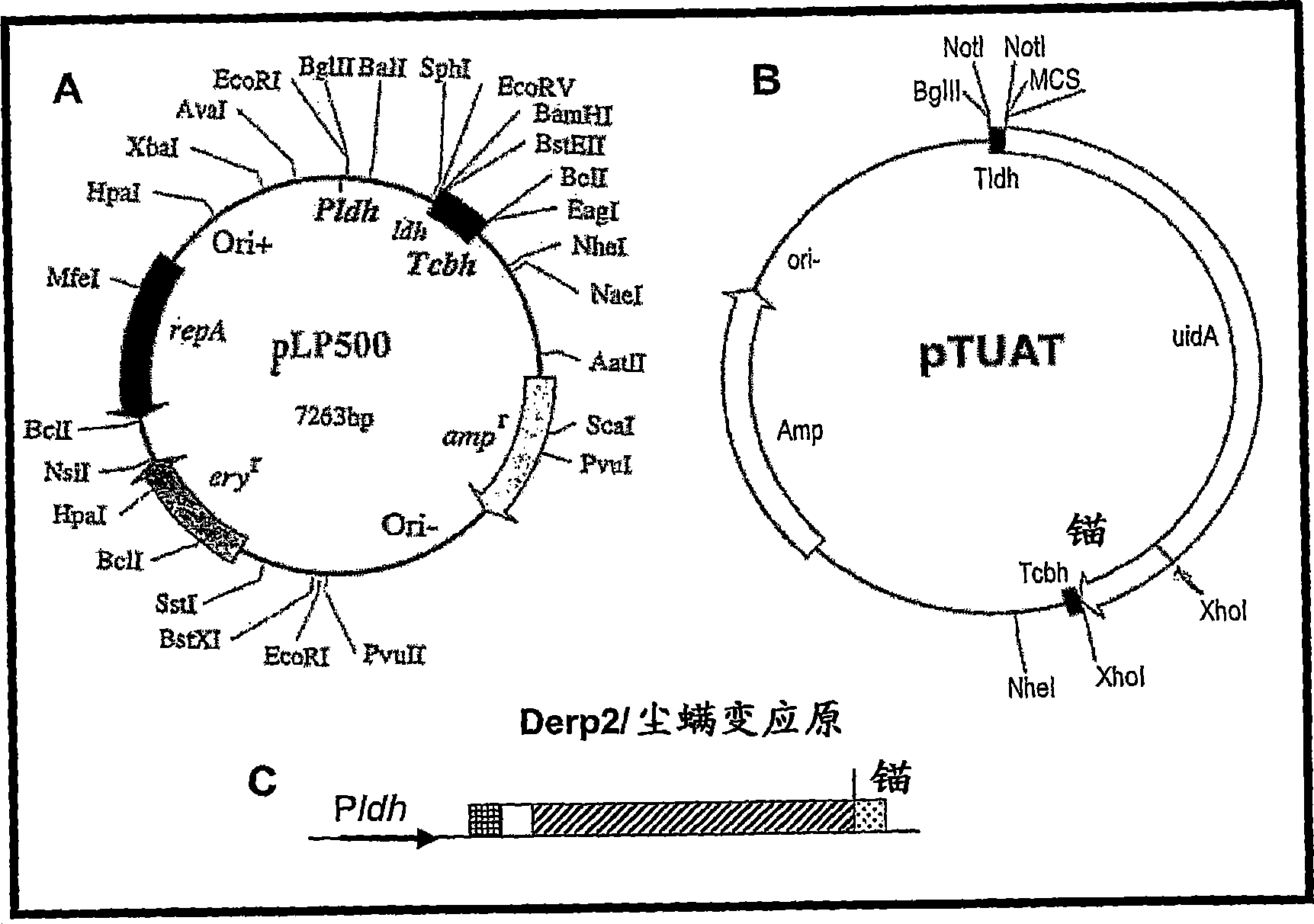
[0179] Example 1: Construction of recombinant Lactobacillus casei expressing enhanced green fluorescent protein (eGFP) using extension high-fidelity DNA polymerase (Boehringer) and synthetic primers BamHI-eGFP / f[5′-CCC CCG GAT CCA gtg agc aag ggc gag gag ctg-3', SEQ ID NO: 3] and eGFP-xhoSac / r[5'-CCC CCC ctc gag CTT GTA CAG CTC GTC CATGCC GAG-3', SEQ ID NO: 4] were analyzed by polymerase chain reaction ( PCR) to amplify the eGFP gene. The resulting PCR products (kits) containing a Bam HI site at the 5' end and an Xho I / Sac I site at the 3' end were then subcloned into pTUAT at Bam HI and Sac I (cf. figure 1 ). The Bam HI / Nhe I fragment containing eGFP-uidA-Tbch was exchanged with the Bam HI / Nhe I fragment expression-secretion vector of pLP500 (refer to figure 1 ).
[0180] The uidA gene was then removed by digestion with Xho I, resulting in the expression construct pLP500-eGFP.
Embodiment 2
[0181] Example 2: Cloning of Der p2 and Blo t5 genes into a Lactobacillus / E. coli shuttle vector
[0182] Using extension high-fidelity DNA polymerase (Boehringer) and synthetic primers, Dp2Bam / f [5'-CCCCCGGATCCAGATCAAGTCGATGTCAAAGATTGTGC-3', SEQ ID NO:5] and Dp2xhoSac / r [5'-CCCCCCGAGCTCCTCGAGATCGCGGATTTTAGCATGAGTAGC-3', SEQ ID NO:6] The 441 bp fragment of Der p2 cDNA was amplified by PCR. The resulting PCR product of the Der p2 gene containing Bam HI and Xho I / Sac I sites at the 5' and 3' ends, respectively, has the sequence: 5'-GGATCC A GAT CAA
[0183] GTC GAT GTC AAA GAT TGT GCC AAT CAT GAA ATC AAA AAA GTT TTG GTA
[0184] CCA GGA TGC CAT GGT TCA GAA CCA TGT ATC ATT CAT CGT GGT AAA CCA
[0185] TTC CAA TTG GAA GCC GTT TTC GAA GCC AAC CAA AAC ACA AAA ACC GCT
[0186] AAA ATT GAA ATC AAA GCC TCA ATC GAT GGT TTA GAA GTT GAT GTT CCC
[0187] CGT ATC GAT CCA AAT GCA TGC CAT TAC ATG AAA TGC CCA TTG GTT AAA
[0188] GGA CAA CAA TAT GAT ATT AAA TAT ACA TGG AAT GTT CCG AAA ATT...
Embodiment 3
[0203] Example 3: Confocal analysis of Lactobacillus casei Shirota-eGFP
[0204] Lactobacillus casei Shirota cells containing the pL500-eGFP construct (L. casei Shirota-eGFP) and the pL500 vector, respectively, were in MRS medium (Difco Laboratories Detroit) containing 5 μg / ml erythromycin at 37° C. 5.0% CO 2 Cultivated in an incubator. When the culture reaches an OD of 0.6 to 1.8 690nm , a total of 0.5 ml was collected and washed twice in PBS (pH 7.4). Cells were resuspended in 1 ml PBS and analyzed under a confocal microscope. Lactobacillus casei Shirota containing the pL500 vector was used as a negative control.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com