Anthryl derivative and preparation thereof
A technology of anthracenyl derivatives and anthracenisoalcohol, which is applied in the field of organic electroluminescence, can solve problems such as poor stability, steric hindrance, or unsaturated coordination number, and achieve high stability, extended service life, and improved color saturation degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to better understand the content of the present invention, the technical solutions of the present invention will be further described through specific examples below, but the examples do not limit the present invention.
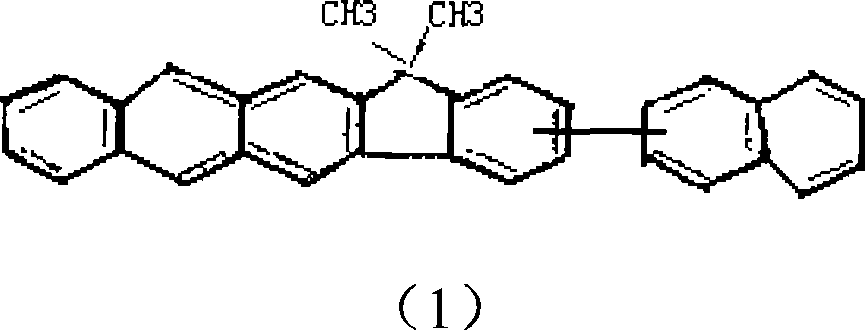
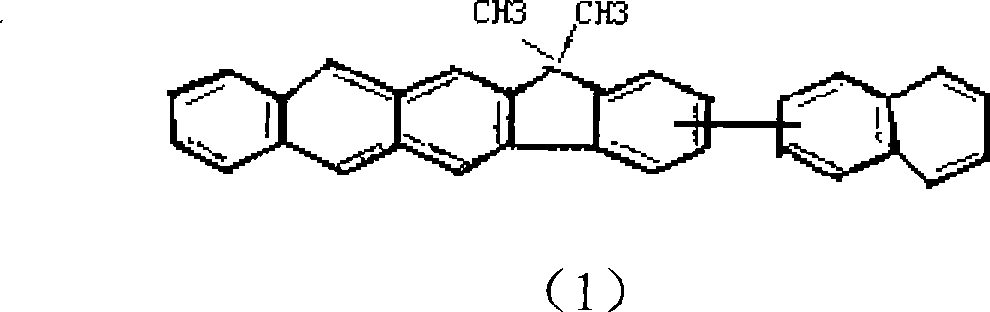
[0029] Take the preparation of 13,13-dimethyl-3-(2-naphthalene)-13 indeno(1,2-b)anthracene as an example:
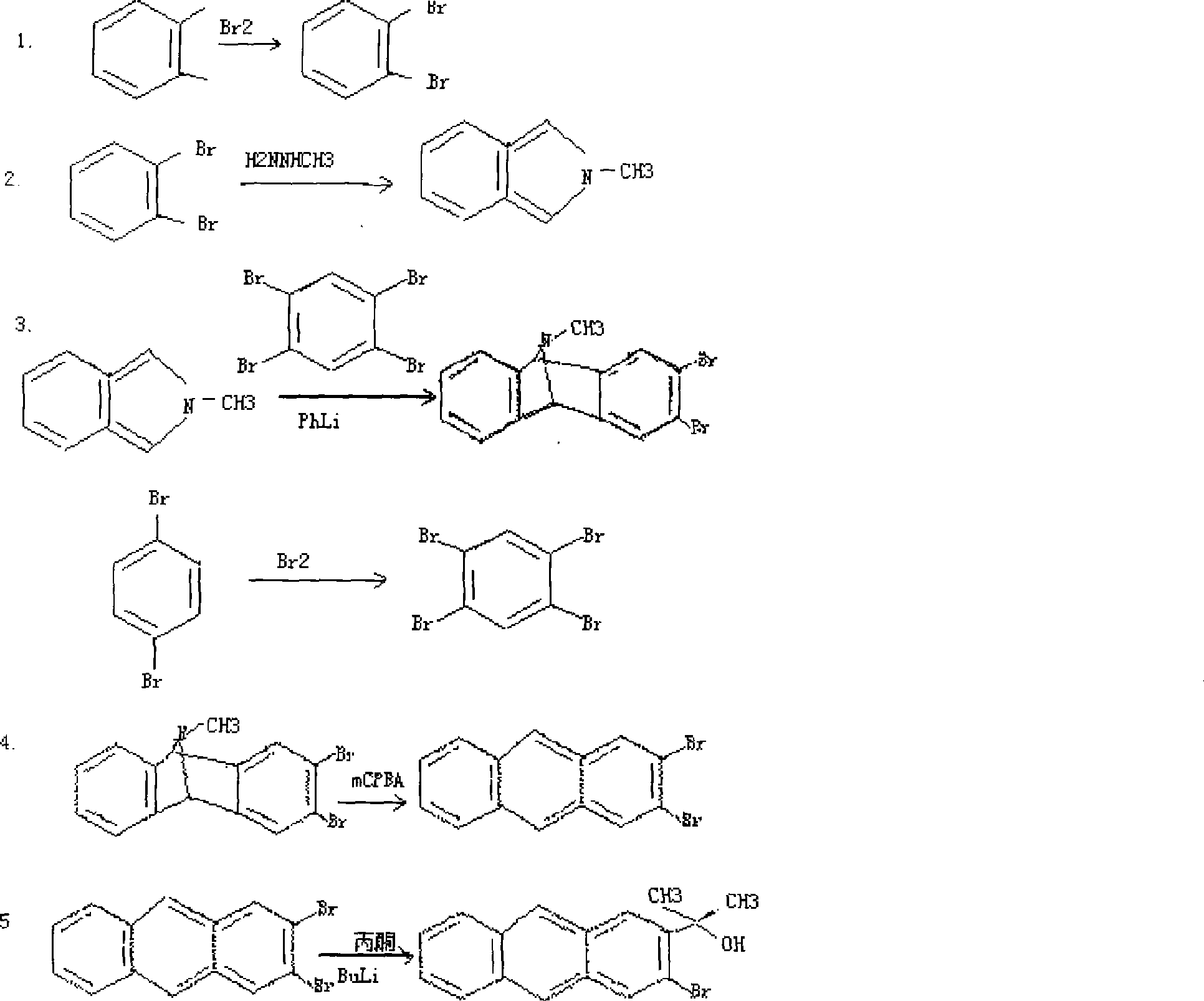
[0030] A step reaction:
[0031] 1. Add catalyst to o-xylene, cool to below 5°C, add bromine dropwise, after dropwise addition, react with light for 20 hours, then wash with 5% sodium hydroxide solution and distilled water successively, after washing, distill to get the product o-Dibromotoluene, the yield of this reaction is about 85%.
[0032] 2. Add dichloromethane and concentrated hydrochloric acid to o-dibromotoluene, add methylhydrazine while stirring, and quickly heat up to 50°C after the reactant is completely dissolved, react for 4 hours, then add sodium nitrite dropwise, and distill to obtain Dry 1-methylisoindole.
[0033] 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com