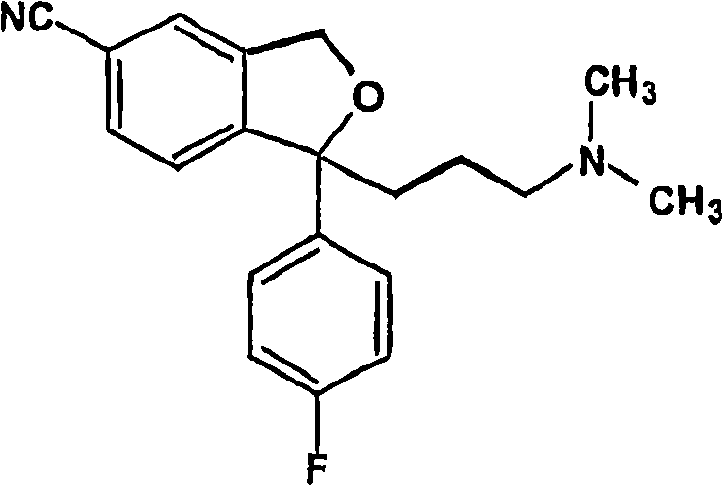
Crystalline composition containing escitalopram oxalate
A technology of escitalopram and oxalate, which is applied in the field of crystalline compositions containing escitalopram oxalate, and can solve the problems of expensive equipment, complexity, and large energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Removal of hydroxyl-containing impurities by succinic anhydride
[0095] R- and S-carboxylates containing 0.6% of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile A mixture of phthalopram (55,5 g) was dissolved in anhydrous toluene (145,0 g). Succinic anhydride (0,5 g) was added to the solution, the mixture was stirred at 45° C. for 120 minutes, water (230 ml) and ammonia (25% by weight) (3 ml) were added (pH=10,5-11,0). The phases were separated and the toluene phase was washed with water (3x120ml). Evaporation of the toluene phase yielded 53,0 g (95%). The product contains 0,06% of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile.
Embodiment 2
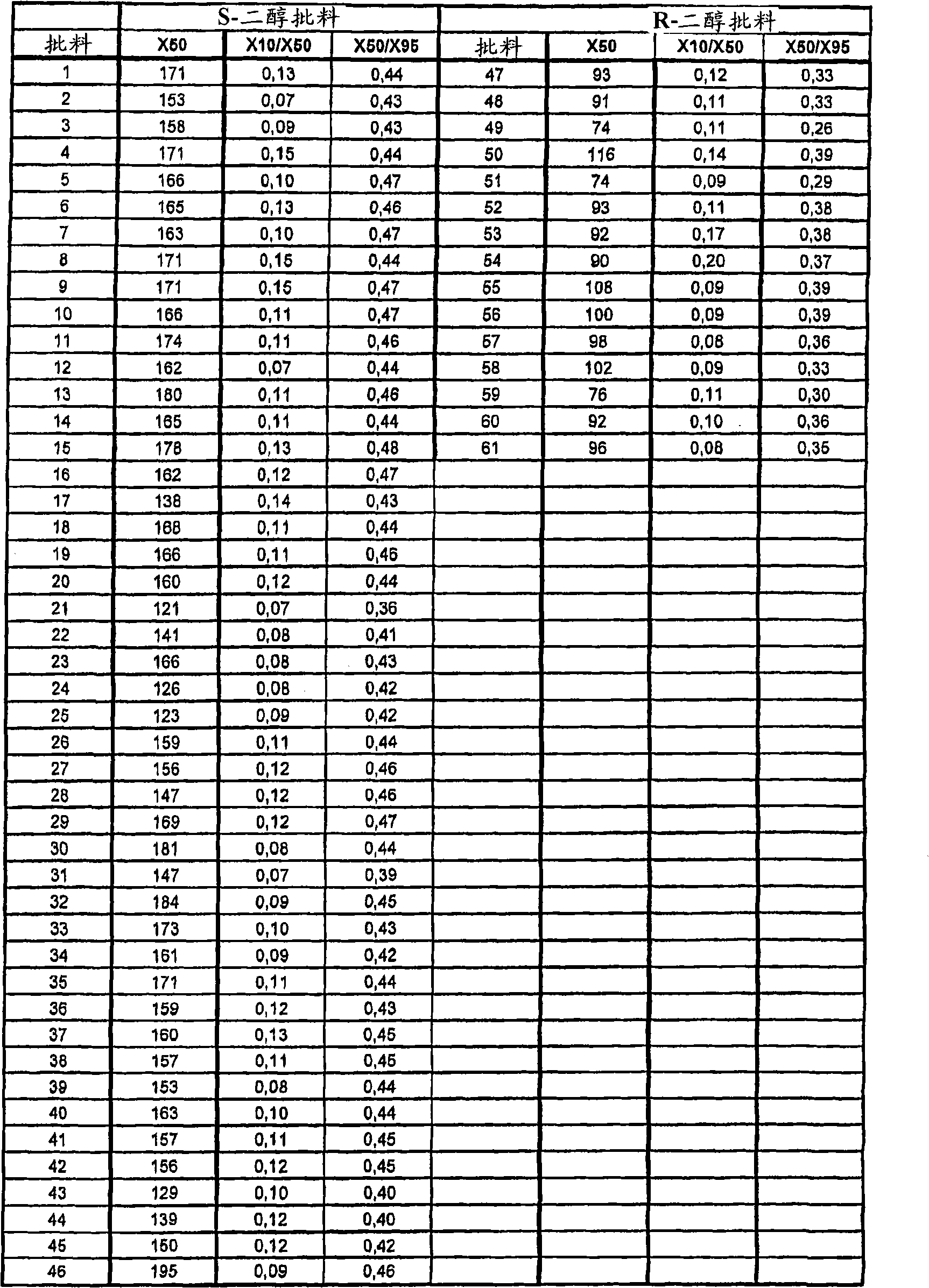
[0096] Example 2: Crystallization of escitalopram oxalate on a production scale
[0097] A number of crude batches of escitalopram oxalate were recrystallized on a production scale according to the procedure described below. Each batch includes:
[0098] a) The preparation of escitalopram is as follows: according to WO03 / 000672, the acidic ring-closing reaction of the R-shaped diol precursor is carried out, and then according to the method described in Example 1, impurities containing hydroxyl groups are removed by a production-scale method, Racemic citalopram and escitalopram were then isolated as described in WO03 / 000672. These batches contained Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, usually relative to escitalopram 0.05% (w / w). These batches are referred to as R-diol batches.
[0099] b) Preparation of escitalopram by ring closure from S-shaped diol precursor via activated ester form under basic conditions as described in US Pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com