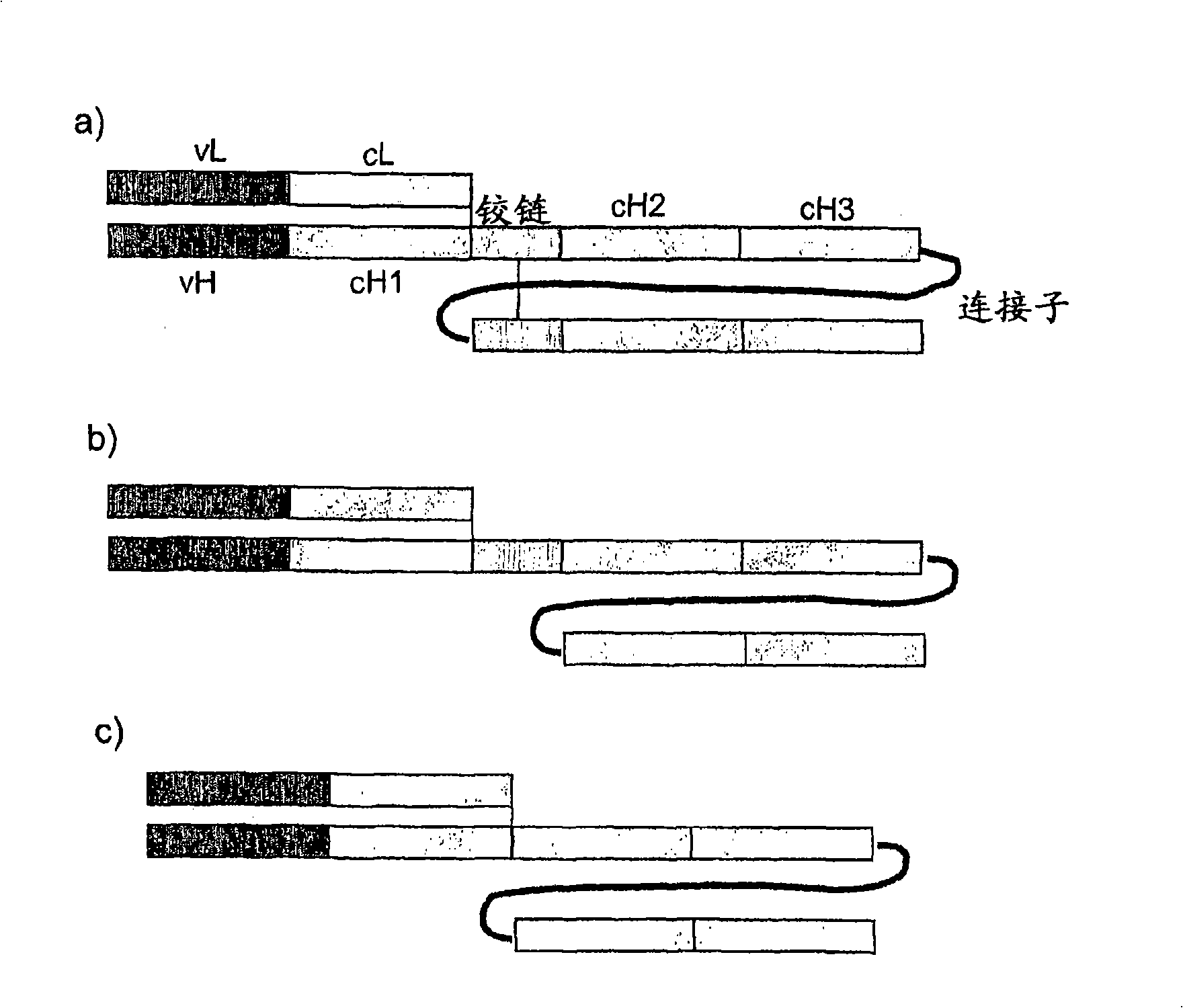
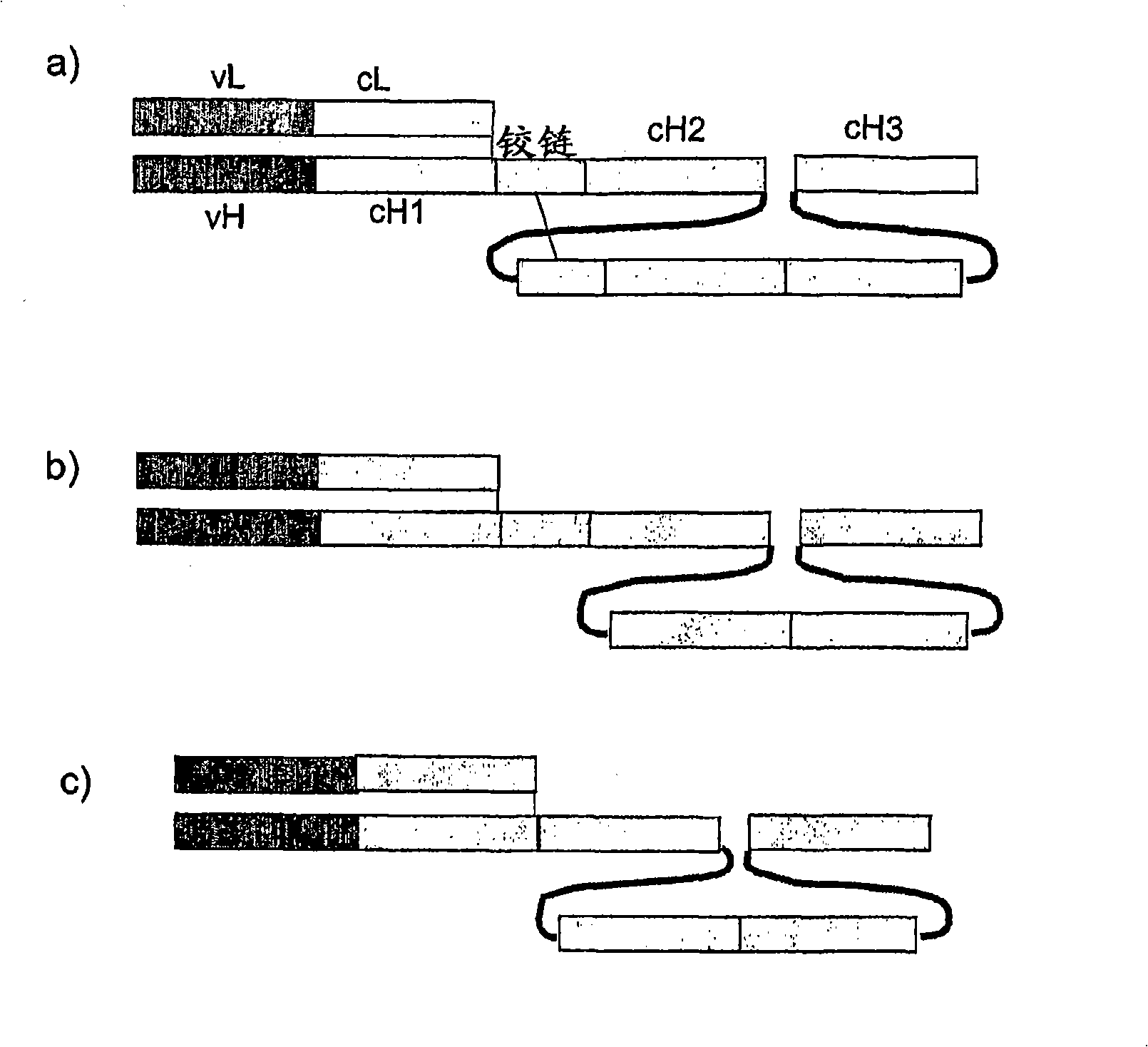
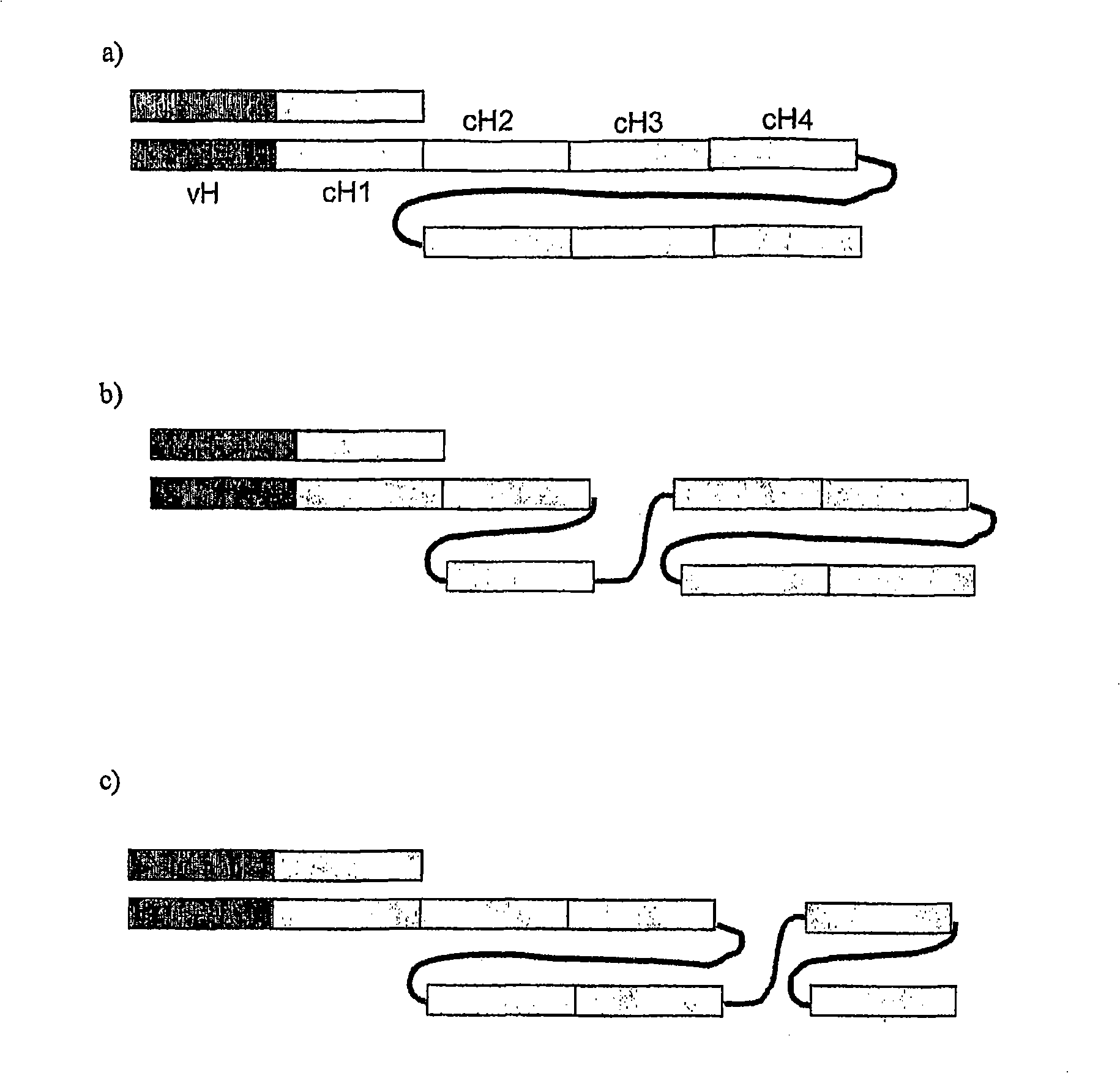
Single chain FC polypeptides
A single-chain and polypeptide-chain technology, applied in the field of single-chain Fc polypeptides, can solve problems such as low-yield chromatographic procedures and complicated preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Three murine single-chain Fc polypeptides were designed containing a biologically active molecule at their N-terminus, wherein the biologically active molecule is an antibody Fab fragment. The variable regions of the Fab fragments are derived from the murine antibody Mox46, which binds cell surface protein antigens. The Fc domains are derived from murine IgG2a and three different versions of these domains are shown below. Linker sequences are underlined. Hinge sequences are italicized and sequences forming part of the linker are italicized and underlined.
[0163] version 1 SEQ ID NO: 88 (as in figure 2 in the form shown in b). Cysteine in the hinge between CH1 and CH2 has been replaced by serine.
[0164] EPRGPTIKPSPPSKSPAPNLLGGPSVFIFPPKIKDVLMISLSPIVTCVVVDVSED
[0165] DPDVQISWFVNNVEVHTAQTQTHREDYNSTLRVVSALPIQHQDWMSGKEFK
[0166] CKVNNKDLPAPIERTISKPK GGGGSGGGGSGGGGSGGGGSGGGGS APNLLG
[0167] GPSVFIFPPKIKDVLMISLSPIVTCVVVDVSEDDPDVQISWFVNNVEVHTAQT
[0168] ...
Embodiment 2
[0198] Example 2: Antigen Binding
[0199] The ability of single-chain Fc polypeptides (versions 1 and 3) to bind antigen was compared to that of the same antibody variable region (from the MOX46 antibody) expressed in the murine IgG1 framework and an irrelevant IgG. Recombinant NSO cells (5×10 5 ) and 100 μl of single-chain Fc polypeptide were incubated at 4° C. for 30 minutes. The control was MOPC21, titrated with a 1 / 3 dilution of said MOPC21 at 5 μg / ml. Titrations were performed with 1 / 3 dilutions of MOX46 IgG and single chain Fc polypeptide constructs. Wash the cells twice with Dulbecco's PBS containing 5% FCS and 0.1% sodium azide, then add 100 μl of PE-labeled (Jackson) antibodies against mouse heavy and light chains diluted at 1 / 250, at 4°C Incubate for 30 minutes. Cells were washed once more before analysis by flow cytometry.
[0200] Like MOX46 IgG, both single chain Fc polypeptide constructs tested bound antigen (1.1 and 3.1). Irrelevant controls did not bind ...
Embodiment 3
[0203] Example 3: Fc receptor binding
[0204] The ability of single chain Fc versions 1 and 3 to bind the Fc receptors CD64 (FcyRII) and CD32 (FcyRI) was determined by BIAcore. CD64 and CD32 were immobilized (approximately 1000 RU each) on flow cells 2 and 3 of a standard CM5 Biacore chip, respectively, using amino-coupling chemistry. Set flow cell 1 as the reference flow cell to check for background binding. The single chain Fc protein was then injected into the sequence covering the chip to check the binding activity. All samples were run undiluted and diluted 1:2 and 1:5. All samples showed no significant background binding.
[0205] Both versions 1 and 3 of the single chain Fc were found to bind both CD64 and CD32.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com