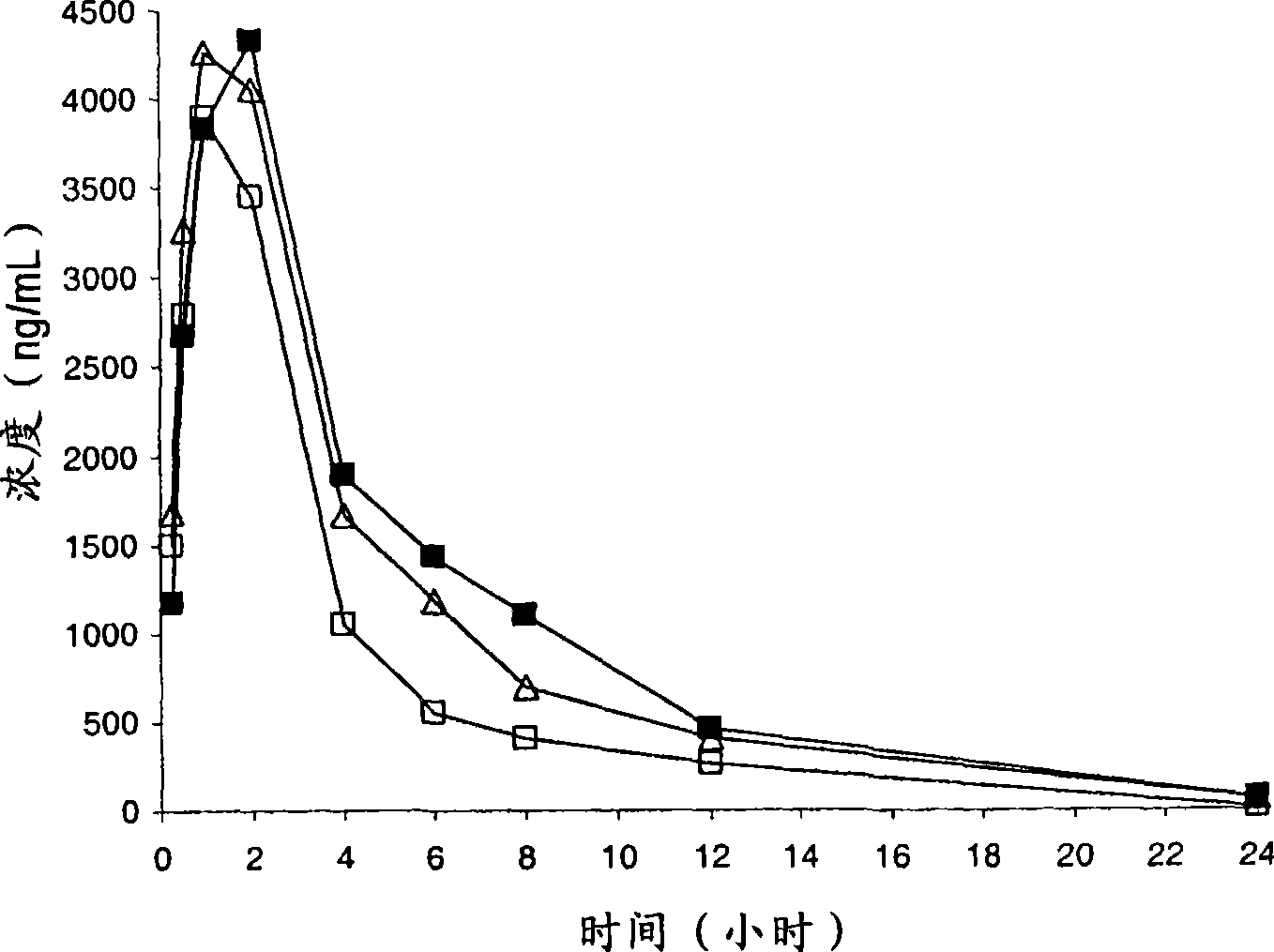
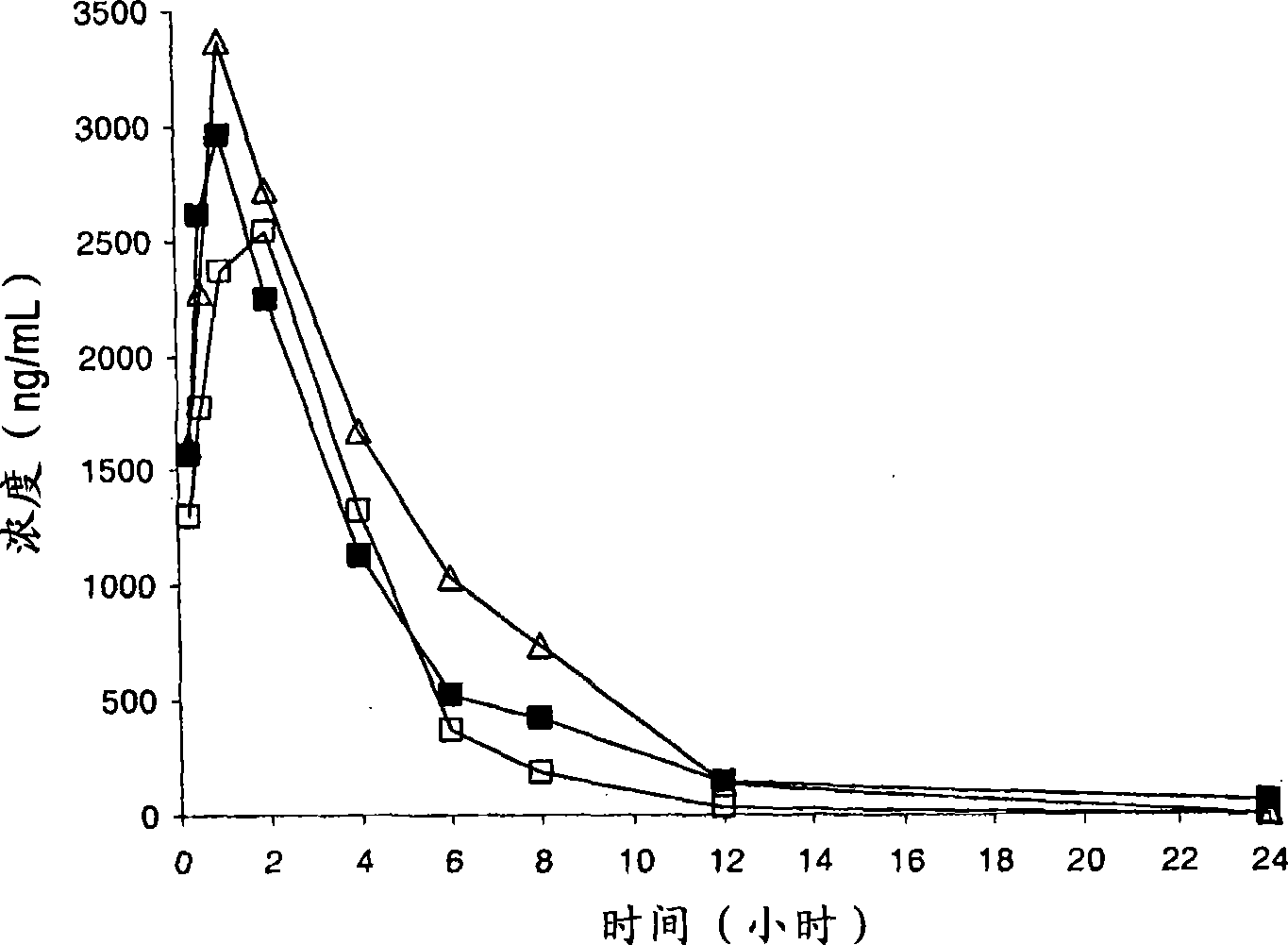
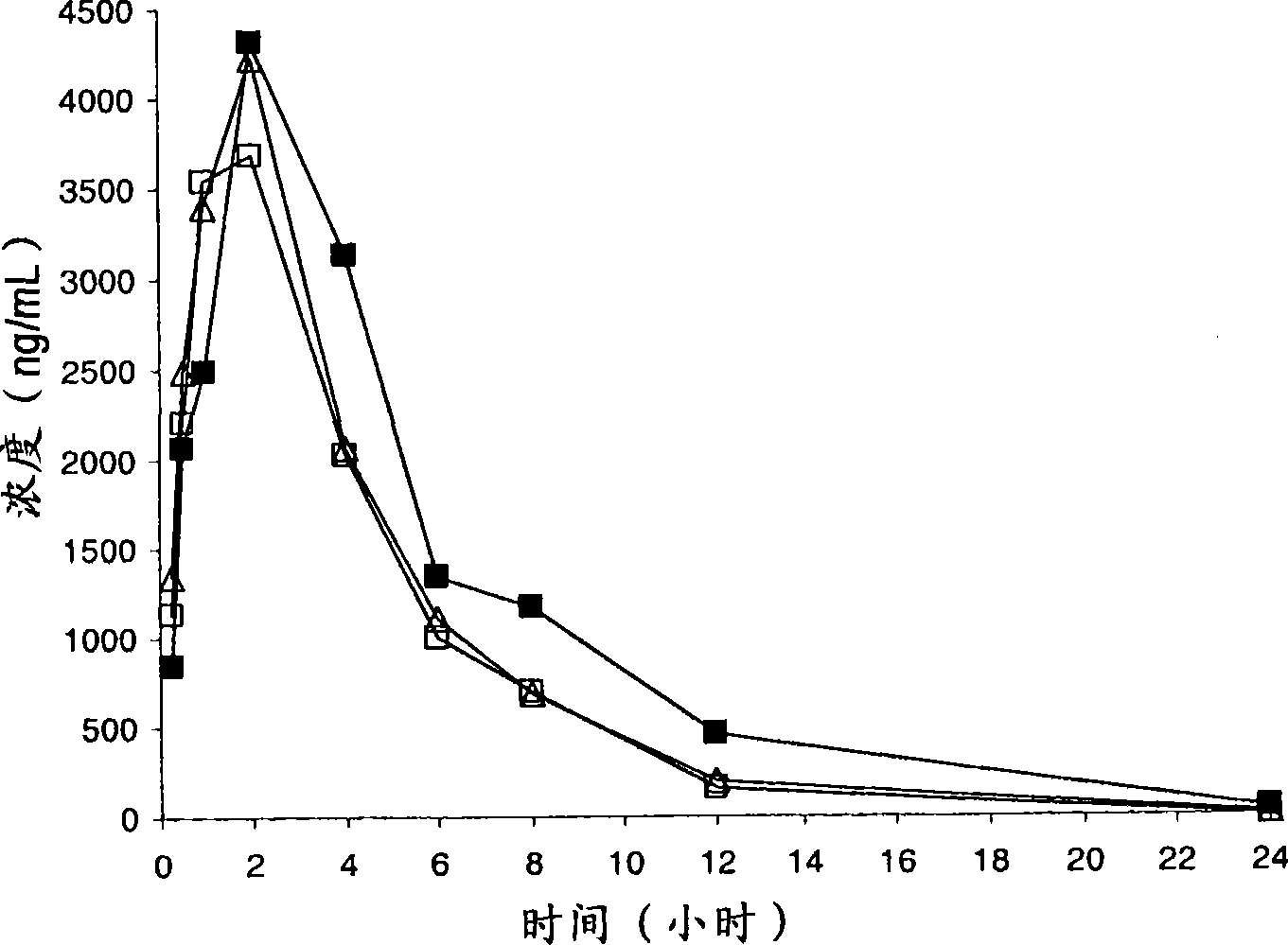
Compounds having CRTH2 antagonist activity
A compound, CH2 technology, applied in the direction of organic active ingredients, organic chemistry, medical preparations containing active ingredients, etc., can solve problems such as low efficacy and not optimal pharmacokinetic distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Example 1: Preparation of 2-(5-fluoro-2-methyl-3-(2-(benzenesulfonyl)benzyl)-1H-indol-1-yl)acetic acid (compound 1)
[0204] Start with commercially available sodium salt of benzenesulfinate and 2-fluorobenzaldehyde.
[0205] a) Process I.(S N Ar) to produce 2-(phenylsulfonyl)benzaldehyde
[0206] To a solution of 2-fluorobenzaldehyde (5.00ml, 47.6mmol) in dimethylsulfoxide (45ml) was added benzenesulfinic acid sodium salt (8.60g, 52.4mmol), and the resulting mixture was heated to 100°C. Benzene sulfinate is dissolved by heating. The solution was heated at 100°C for 3 days. The reaction solution was cooled to room temperature, and water (50ml) was added. The mixture was extracted with ethyl acetate, the combined organic extracts were washed with saturated brine, washed with MgSO 4 Dry and concentrate in vacuo. The crude material was purified by flash chromatography on silica gel eluting with 25% ethyl acetate:petroleum ether (40-60°C) to 33% ethyl acetate:petroleu...
Embodiment 2
[0213] Example 2: Preparation of 2-(3-(2-(4-chlorobenzenesulfonyl)benzyl)-5-fluoro-2-methyl-1H-indol-1-yl)acetic acid (compound 2)
[0214] Start with commercially available 2-(4-chlorophenylthio)benzaldehyde.
[0215] a) Process J. (direct oxidation) to produce 2-(4-chlorobenzenesulfonyl)benzaldehyde
[0216] To a solution of 2-(4-chlorophenylthio)benzaldehyde (2.00 g, 8.00 mmol) in dichloromethane (20 mL) was added m-chloroperoxybenzene in portions over 15 minutes at 0°C Formic acid (77% max, 5.40 g, 24.17 mmol) was then warmed to room temperature and stirred for 2 hours. Aqueous sodium metabisulfite solution was added carefully until bubbling ceased. The solution was extracted with dichloromethane, the combined organic extracts were washed with NaOH (1 N), followed by saturated brine, washed with MgSO 4 Drying and concentration in vacuo yielded a white solid (1.05 g, 3.74 mmol, 46%). δ H (300MHz, d 6 -DMSO) 10.69 (1H, s, CHO), 8.25-8.18 (1H, m, Ar), 8.07-8.00 (2H, m, ...
Embodiment 3
[0220] Example 3: Preparation of 2-(5-fluoro-3-(2-(4-fluorobenzenesulfonyl)benzyl)-2-methyl-1H-indol-1-yl)acetic acid (compound 3)
[0221] a) Process A.(S N Ar) to produce 2-(4-fluorophenylthio)benzaldehyde
[0222] in N 2 Next, to 4-fluorobenzenethiol (0.86ml, 8.06mmol) and K 2 CO 3 (2.50g, 18.12mmol) in DMSO suspension (5ml) was added 2-fluorobenzaldehyde (1.00g, 8.06mmol), and the mixture was heated at 100°C for 3 hours. The reaction solution was cooled to room temperature, and water (20ml) was added. The mixture was extracted with ethyl acetate, the combined organic extracts were washed with saturated brine, washed with MgSO 4 Drying and concentration in vacuo yielded a yellow solid (1.20 g, 5.17 mmol, 64%).
[0223] δ H (300MHz, d 6 -DMSO) 10.23 (1H, s, CHO), 7.98 (1H, dd, J 7.3 and 1.7Hz, Ar), 7.63-7.49 (3H, m, Ar), 7.45-7.32 (3H, m, Ar) and 6.88 (1H, doublet, J7.7Hz, Ar).
[0224] b) Process B. (Aldehyde protection) to produce (2-(dimethoxymethyl)phenyl)(4-fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com