Method for supporting, regulating and controlling amplification of hematopoietic stem/progenitor cell in vitro with microencapsulation osteoblast in hypoxia condition
A technology for osteoblast and in vitro expansion, applied in biochemical equipment and methods, bone/connective tissue cells, animal cells, etc., can solve problems such as unsatisfactory cell expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Co-cultivation of microencapsulated osteoblasts and umbilical cord blood HSPCs under normoxia
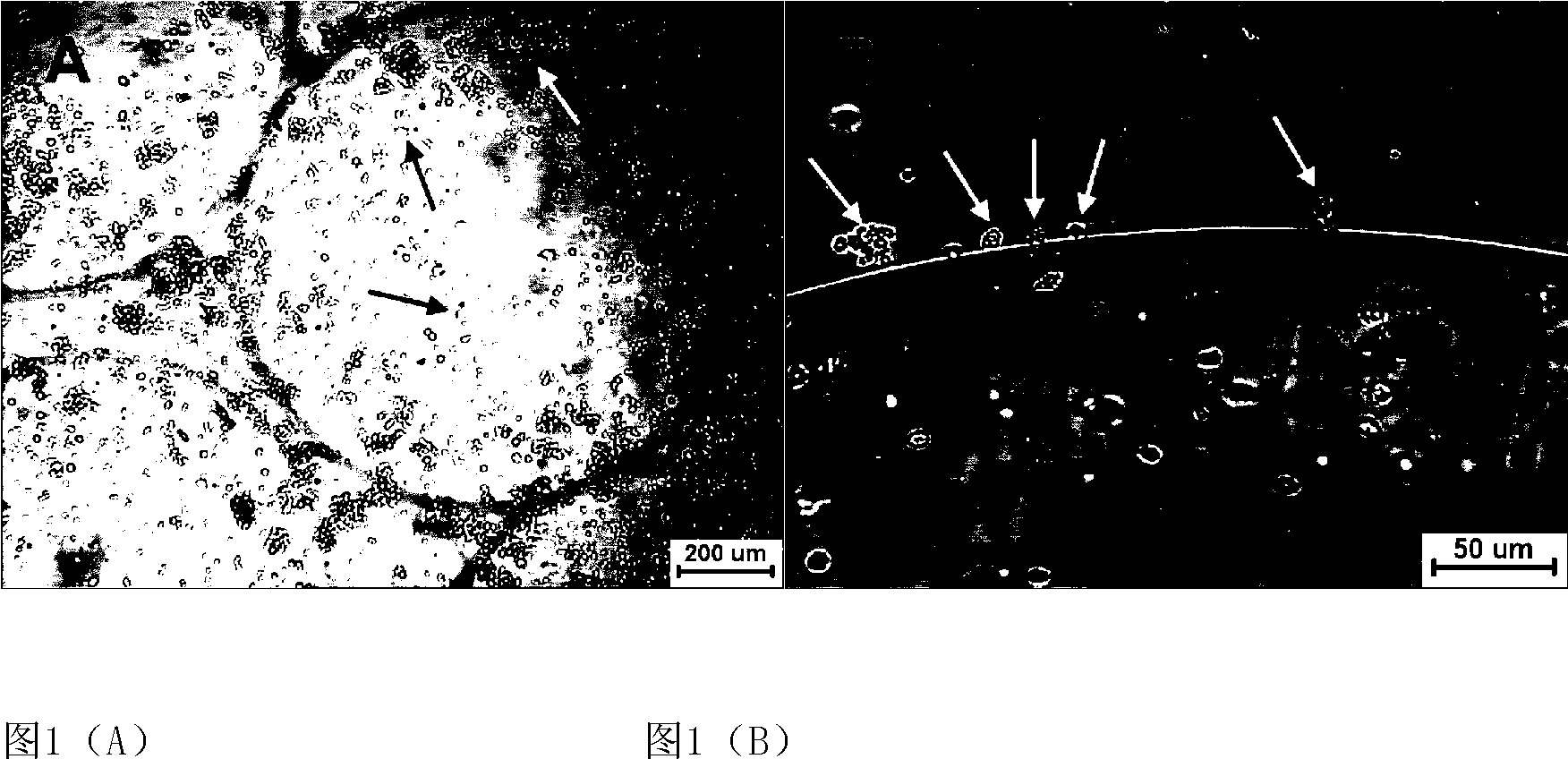
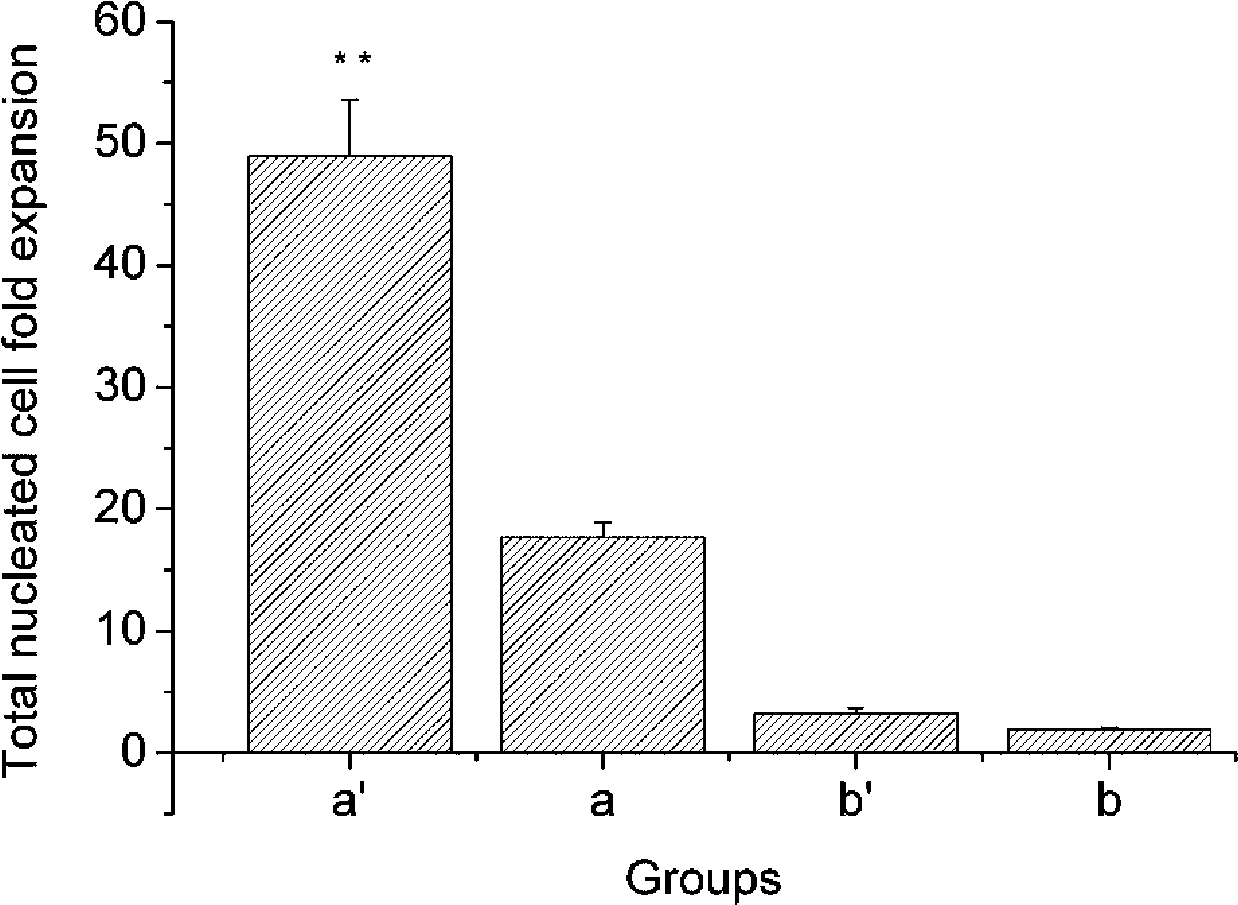
[0044] The embedding density of osteoblasts was 8×10 5 cells / mL, 1mL was added to each well, and three wells were paralleled in each group, a total of 2 groups. One group was used for co-culture with hematopoietic cells, and the other group was used as a control without hematopoietic cells. The diameter of the microbeads is 0.5 mm. The co-culture group with osteoblasts in a normoxic environment is marked as a, and the single culture of cord blood mononuclear cells is marked as b. Adjust the freshly isolated umbilical cord blood total nucleated cell density to 2×10 5 cells / mL, inoculated in a 6-well plate containing human osteoblast microbeads, added 2 mL to each well, and paralleled 3 wells in each group. Serum-free IMDM was used as the medium, and a small amount of growth factors were added, including 2.4ng / mLIL-3, 20ng / mLSCF, 10ng / mLFL, 2.0ng / mLGM-SCF and 3.2n...
Embodiment 2
[0047] Example 2 Co-cultivation of osteoblast microgel beads with different diameters and umbilical cord blood HSPCs
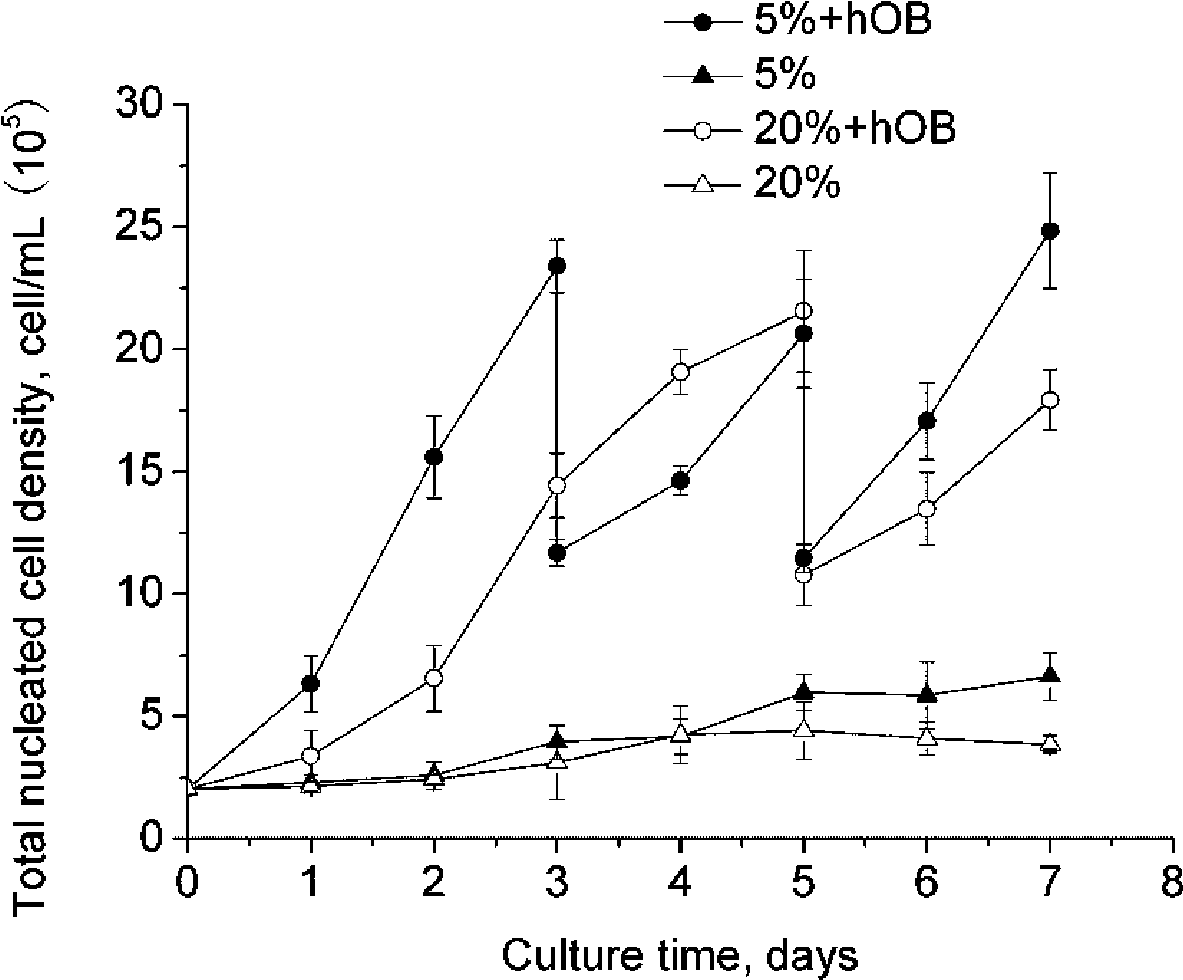
[0048] Adjust the cord blood mononuclear cell density to 2×10 5 cells / mL, seeded in a 6-well plate, and added 3 mL of cell suspension to each well. Then add 1 mL of osteoblasts to each well at a density of 5 × 10 5 cells / mL microgel beads. The ratio of osteoblasts to umbilical cord blood hematopoietic cells was 5:6. placed in a hypoxic incubator (5% O 2 , 20% CO 2 , 37°C). The 0.5mm microgel bead group is marked as A, and the 2mm microbead group is marked as B. Serum-free IMDM was used as the co-culture medium, and a small amount of growth factors were added according to the standard in Example 1. Co-cultivate for 7 days, gently blow off the cells before sampling every day, so that the signal factors and hematopoietic growth factors secreted by osteoblasts are evenly dispersed, take 0.2mL cell suspension from each well for various tests, and add the sam...
Embodiment 3
[0051] Example 3 Co-cultivation of microencapsulated osteoblasts and umbilical cord blood HSPCs under optimized conditions
[0052] The embedding density of osteoblasts was 8×10 5 cells / mL, 1mL was added to each well, and three wells were paralleled in each group, a total of 2 groups. One group was used for co-culture with hematopoietic cells, and the other group was used as a control without hematopoietic cells. The diameter of the microbeads is 0.5 mm. The group co-cultured with human osteoblasts in a hypoxic environment was marked as a', and the group cultured with umbilical cord blood mononuclear cells alone was marked as b'. Adjust the total nucleated cell density of freshly isolated umbilical cord blood to 2×10 5 cells / mL, inoculated in a 6-well plate containing human osteoblast microbeads, added 2 mL to each well, and paralleled 3 wells in each group. Serum-free IMDM was used as the medium, and a small amount of growth factors were added according to the standard in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com