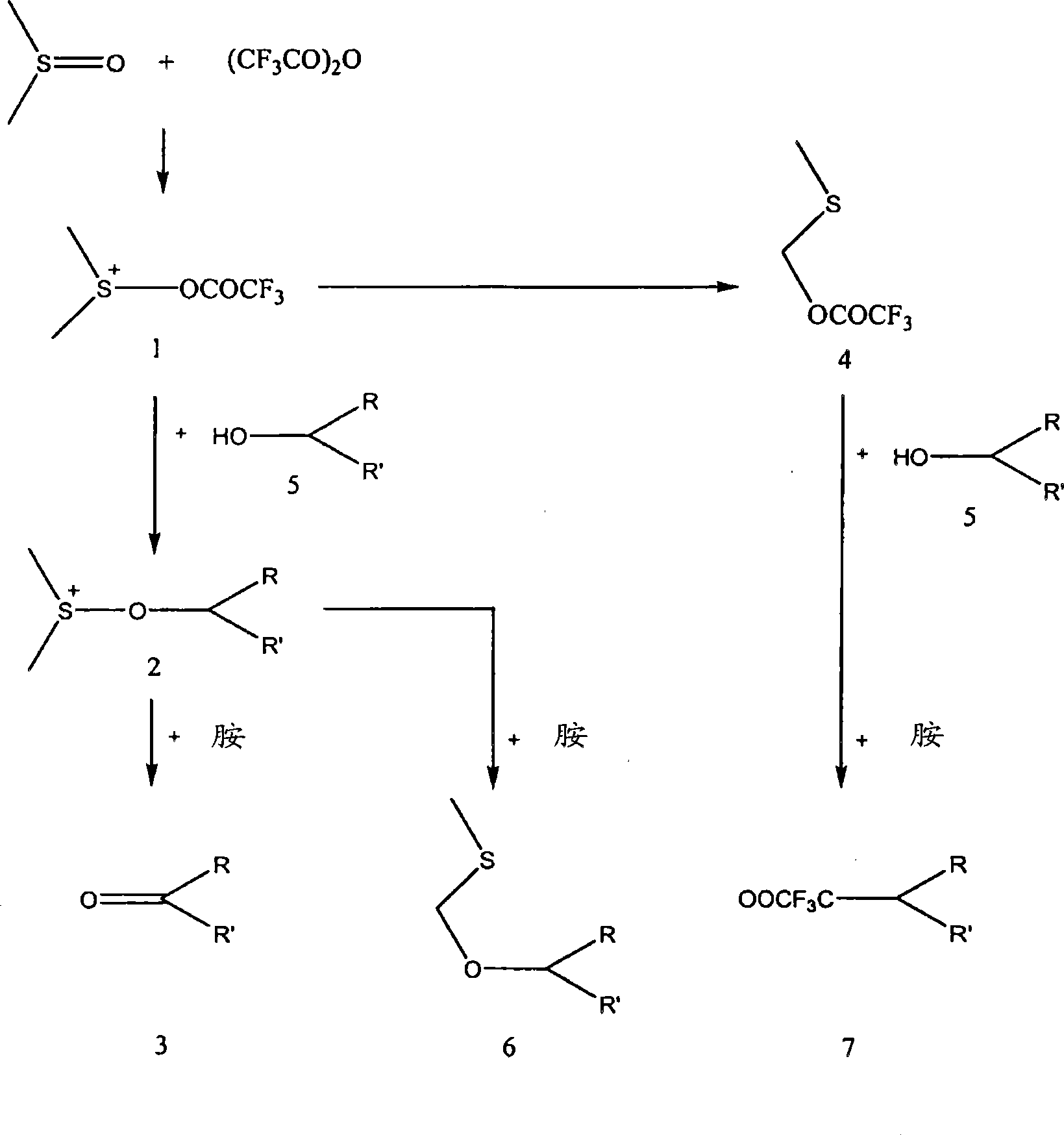
A method for the targeted execution of a reaction taking place alongside several competing chemical reactions.
A chemical reaction and competitive technology, used in organic chemistry, oxidation to prepare carbonyl compounds, etc., can solve problems such as cost, and achieve the effect of simplifying demand and minimizing temperature-dependent unstable intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Two-step continuous processing
[0032] Solution 1: contained in CH 2 Cl 2 TFAA (0.6M); flow rate: 10ml / min
[0033] Solution 2: contained in CH 2 Cl 2 cyclohexanol (0.5M) and DMSO (1M); flow rate: 10ml / min
[0034] Solution 3: contained in CH 2 Cl 2 tributylamine (0.73M); flow rate: 20ml / min
[0035] This continuous process is carried out in a 2-step microreactor system. In step 1, solutions 1 and 2 were mixed in a micromixer with a residence time of 2 seconds. The reaction was carried out simultaneously at temperatures of -20°C and +20°C. Subsequently, this mixture was mixed with solution 3 in a micromixer (step 2) with a residence time of 4 seconds and at the same temperature as in step 1. The whole solution was then allowed to warm to room temperature and allowed to stand for several minutes before analyzing the mixture by gas chromatography (GC). The yield of cyclohexanone was obtained in 91%.
Embodiment 2
[0036] Example 2: One-step continuous treatment with batch ammonolysis
[0037] Solution 1: contained in CH 2 Cl 2 TFAA (0.6M); flow rate: 15ml / min
[0038] Solution 2: contained in CH 2 Cl 2 cyclohexanol (0.5M) and DMSO (1M); flow rate: 15ml / min
[0039] Solution 3: contained in CH 2 Cl 2 tributylamine (0.73M); flow rate: 30ml / min
[0040] In the continuous process, solutions 1 and 2 were mixed with a micro-mixer with a residence time of 1.5 seconds (step 1). The reaction was carried out simultaneously at temperatures of -20°C and +20°C. The reaction mixture was then transferred to a conventional reaction vessel (5 sec residence time) via a non-thermostatic tube (step 2). Simultaneously, solution 3 was pumped into the vessel and mixed routinely for 1.5 minutes at room temperature. A total reaction volume of 90 ml was collected. The productive rate (93%) of cyclohexanone in the present embodiment is suitable with the productive rate of embodiment 1.
Embodiment 3
[0041] Embodiment 3: One-step continuous processing
[0042] Solution 1: contained in CH 2 Cl 2 TFAA (0.3M) and tributylamine (0.73M); flow rate: 40ml / min
[0043] Solution 2: contained in CH 2 Cl 2 cyclohexanol (0.5M) and DMSO (1M); flow rate: 20ml / min
[0044] In the continuous process, solutions 1 and 2 were mixed by a micromixer in a 1-step microreactor system at a temperature of -30 °C. Subsequently, the entire reaction solution was warmed to room temperature and allowed to stand for several minutes before analyzing the mixture by GC. In this treatment, the yield of cyclohexanone was less than 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com