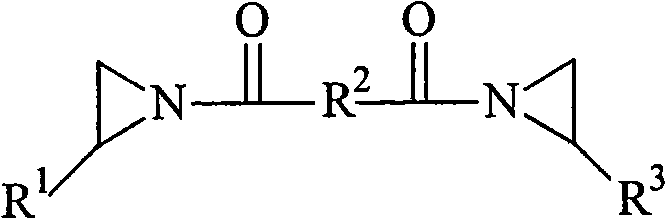2-octyl (meth)acrylate adhesive composition
A technology of adhesives and compositions, applied in the directions of ester copolymer adhesives, adhesive types, adhesives, etc., can solve the problems of unstable price and supply of adhesive products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0088] These examples are for illustrative purposes only, and are not intended to limit the scope of the appended claims. All parts, percentages, ratios, etc. in the examples, as well as in the remainder of the specification, are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company, Milwaukee, Wisconsin unless otherwise indicated.
[0089] Acronym
[0090] abbreviation or trade name
illustrate
2-OA
IOA
AAA
acrylic
VAZO67
Available from DuPont, Wilmington, DE
Commercially available 2,2'-azobis-(2-methylbutyronitrile) radical initiator.
IV
PDI
polydispersity index
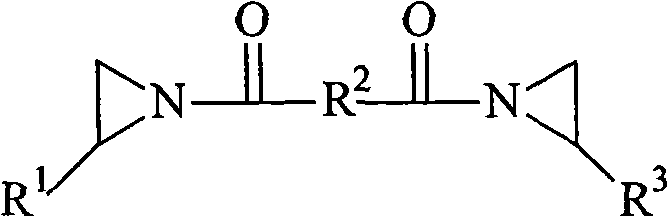
B-212
Bisamide crosslinker, 1,1'-isophthaloyl-bis(2-methylaziridine) (CAS No.
7652-64-4), in solution as a 5% by weight solution of the bisamide in toluene.
THF
Tetrahyd...
preparation example 1
[0096] Preparation example 1: 2-octyl acrylate
[0097]2-octanol (268.51 g, 2.1 moles), AA (183.75 g, 2.6 moles), p-toluenesulfonic acid monohydrate (5.00 g, 26 mmol), toluene (250 g) and phenothiazine (1.0 g) mixture was heated to reflux. Water was separated from the toluene / water azeotrope using a Dean Stark distillation interceptor. After 6 hours at reflux, a total of 37 ml of water was collected in the distillation trap. The reaction mixture was washed with 1 molar aqueous sodium hydroxide (200 mL), and concentrated under reduced pressure. The remaining oil was distilled under reduced pressure (2 mm Hg at 65-67°C) to give the product as a colorless oil. (Yield: 248.6 g)
example 1-2 and comparative example C1-C2
[0099] Part 1: Solution Polymerization
[0100] For Examples 1 and 2, solution copolymerization of 2-OA and AA was accomplished by blending the materials shown in Table 1 in a glass jar, purging with nitrogen for 15 minutes, and sealing the jar. Place the jar in a water bath at 60°C with shaking at 110 rpm for 18 to 20 hours. The same procedure was used for Comparative Examples C1 and C2, except that 2-OA was replaced by IOA. The molecular weight of the resulting polymer (M w ) and PDI were determined by GPC, and intrinsic viscosity (IV) was determined by a #50 viscometer at a solution concentration of 0.5 g / dL in THF. These data are shown in Table 1 below.
[0101] Table 1
[0102] example
2-OA
(gram)
IOA
(gram)
AAA
(gram)
VAZO
67(g)
(gram)
IV
m w
(g / mol)
PDI
1
23.75
----
1.25
0.025
37.5
0.9
3.7×10 5
3.8
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com