A novel population of multipotent cardiac precursor cells derived from human blastocysts derived stem cells
A technology of cells and populations, applied in the field of pluripotent cardiac precursor cell populations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0273] Preparation of feeder cell layer
[0274] Before plating the foreign-free human fibroblast feeder cells, the tissue culture wells were coated with 0.1% recombinant human gelatin (Fibrogen) at room temperature for at least 1 hour. The confluent monolayers of exogenous hFF003 (passages 5 to 8) cells grown in IMDM, 10% human serum and 1% penicillin-streptomycin were then treated with mitomycin C (Sigma) for 2.5 hours. Place the feeder cells treated with mitomycin C on the IVF wells (Becton Dickinson), every 2.89cm in the medium 2 200,000 cells, the medium is based on DMEM (as above), supplemented with 10% (v / v) human serum, 1% penicillin-streptomycin, 1% Glutamax, 0.5 mmol / l β-mercaptoethanol and 1% non-essential amino acids ( Gibco Invitrogen Corporation). Before placing the blastocyst with inner cell cluster cells and cells derived from it or hBS cells, change the medium to DMEM (as above), now instead of supplementing with 20% (v / v) human serum, 10ng / mL Human recombinant bF...
Embodiment 1
[0310] Basal cells derived from undifferentiated hBS cells
[0311] Suspension culture
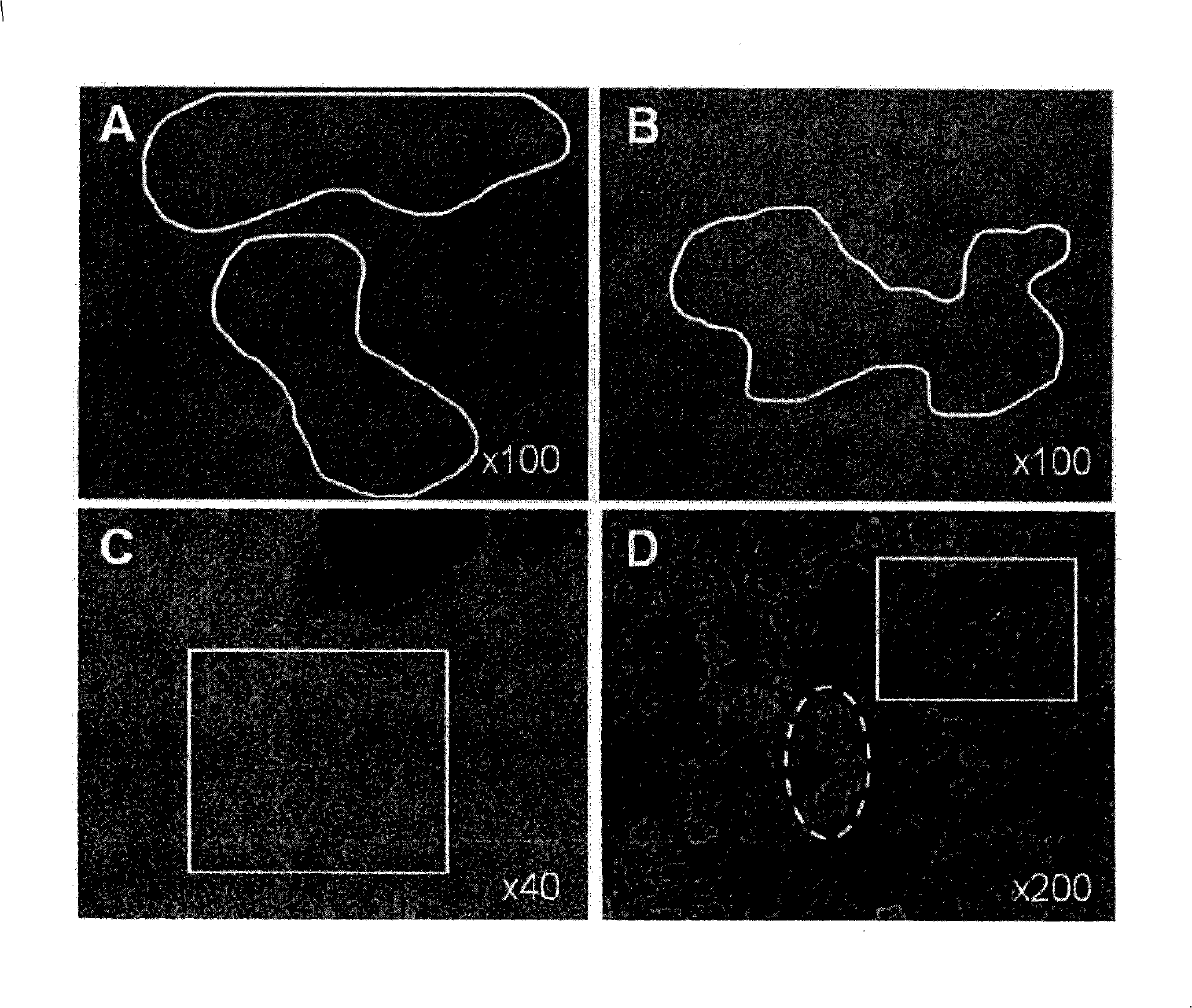
[0312] The undifferentiated hBS cells are maintained and propagated in vitro. On the 4-5 days after the passage, use a stem cell cutting tool to manually separate the undifferentiated hBS cell colonies and place them in the differentiation medium (KO-DMEM, supplemented with 1mM Glutamax, 0.1mM β-MeOH, 1% NEAA, 1% PeST and 20% FBS) in suspension culture. The cells are maintained in suspension for 1-6 days, and then transferred to a gelatin-coated tissue culture dish, resulting in the adhesion of differentiated cell clusters and the continued proliferation and differentiation of cells. Four days after plating, the adherent clusters of differentiated hBS cells appeared as a monolayer of cells. These cells grow in a characteristic dense network that resembles a maze, making the area of the base cell easy to distinguish when using an optical microscope. The morphology of basal cells is similar to...
Embodiment 2
[0316] Characterization of Basal Cells
[0317] Basal cells were derived from undifferentiated hBS cells as described above. They were cultured in differentiation medium (KO-DMEM, supplemented with 1 mM Glutamax, 0.1 mM β-MeOH, 1% NEAA, 1% PeST and 20% FBS) for up to 37 days. Cells were fixed in PFA at different time points and assessed by immunohistochemistry (Noaksson et al. Stem Cells 2005). The antigenic markers used are vimentin, desmin, α-striated muscle actin and short tail protein. Basal cells express α-striated muscle actin and short tail protein ( Picture 9 ) But does not express vimentin and desmin. The cell lines used are SA002, LOTAL002 (passage 26 times); SA002.5, LOTBE002.5 (passage 32 times) and SA461, LOTBF461 (passage 33 times).
[0318] Table I summarizes the results obtained by IHC analysis of basal cells.
[0319] Table I
[0320] Antigenic marker
Days of differentiation *
The result
Antibody information
25
Negati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com