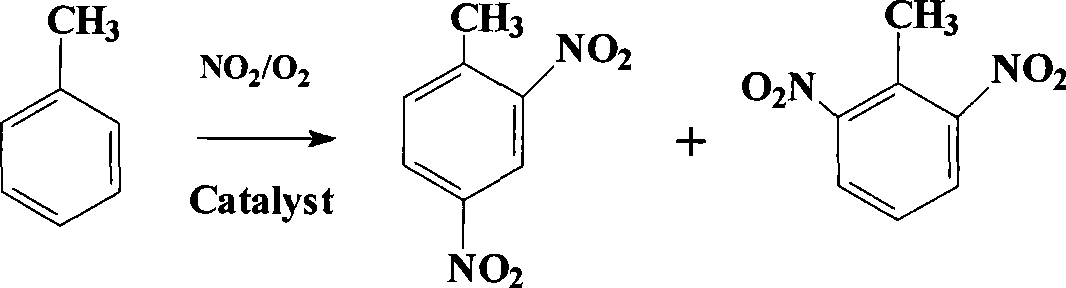
Preparation method for dinitrotoluene
A technology for dinitrotoluene and toluene, applied in the field of preparation of dinitrotoluene, can solve the problems of high cost and limit the industrial application of the Kyodai method, and achieve the effects of less three wastes, convenient recovery and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 46g of toluene into the autoclave, and then add 0.46g of Hβ molecular sieve and 184g of liquid nitrogen dioxide respectively. After the cover is sealed, nitrogen is introduced, and the pressure is tested to confirm that the autoclave does not leak. Then the pressure was relieved and oxygen was introduced to keep the pressure of the reactor at 5MPa. Turn on the stirrer, 400 revolutions per minute, turn on the circulating cooling water, and keep the reaction temperature at 80°C basically unchanged. After stirring for 0.5h, close the oxygen inlet valve, slowly release the gas in the reactor, and ventilate with nitrogen. The exhaust gas is emptied after being absorbed by lye. Stop stirring, then transfer the reaction material to a 500mL glass beaker, filter, wash the filter residue with water, and recover the catalyst. After neutralizing the filtrate, pour it into an equal volume of ice water, and immediately a solid precipitates out. Let it stand overnight, filter, wash ...
Embodiment 2
[0022] 46g toluene, the catalyst is changed to HZSM-5, the mass of HZSM-5 is 6.9g, the liquid nitrogen dioxide is changed to 69g, the reaction pressure is changed to 0.1MPa, the reaction temperature is changed to 20°C, and the reaction time is changed to 10h. Other reactions and post-treatments are the same as in Example 1. The yield of dinitrotoluene was 90.1% (calculated as toluene), and the ratio of 2,4-dinitrotoluene and 2,6-dinitrotoluene was 33:1. After recrystallization, 79.3 g of 2,4-dinitrotoluene was obtained.
Embodiment 3
[0024] 46g toluene, the catalyst is changed to HY, the mass of HY is 4.6g, the liquid nitrogen dioxide is changed to 46.0g, the reaction pressure is changed to 0.5MPa, the reaction time is changed to 10h, and the amount of ice water is changed to 10 of the filtrate volume obtained from the filtration of the reactant. The other reactions and post-treatments are the same as in Example 1. The yield of dinitrotoluene was 90.3% (calculated as toluene), and the ratio of 2,4-dinitrotoluene and 2,6-dinitrotoluene was 33:1. After recrystallization, 79.5 g of 2,4-dinitrotoluene was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com