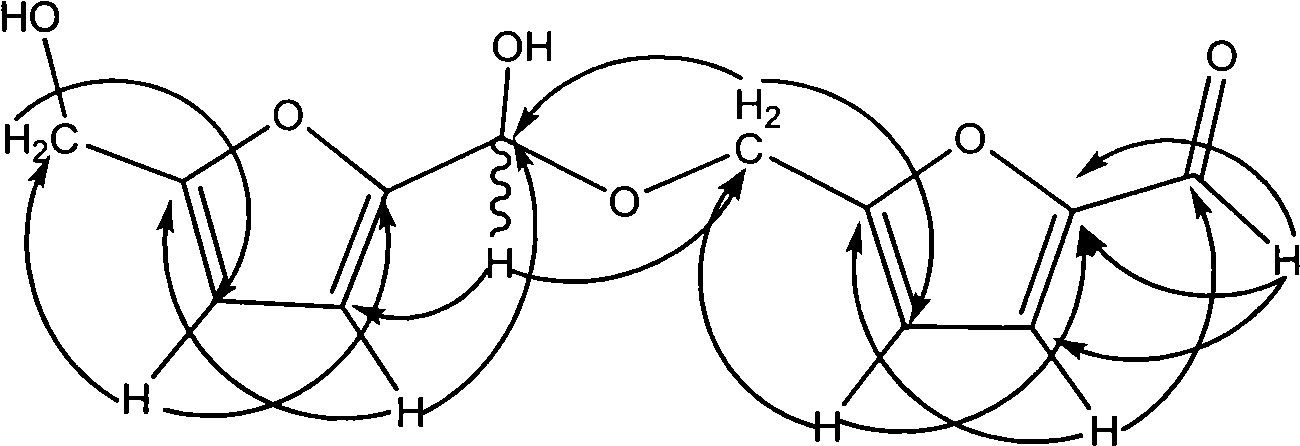
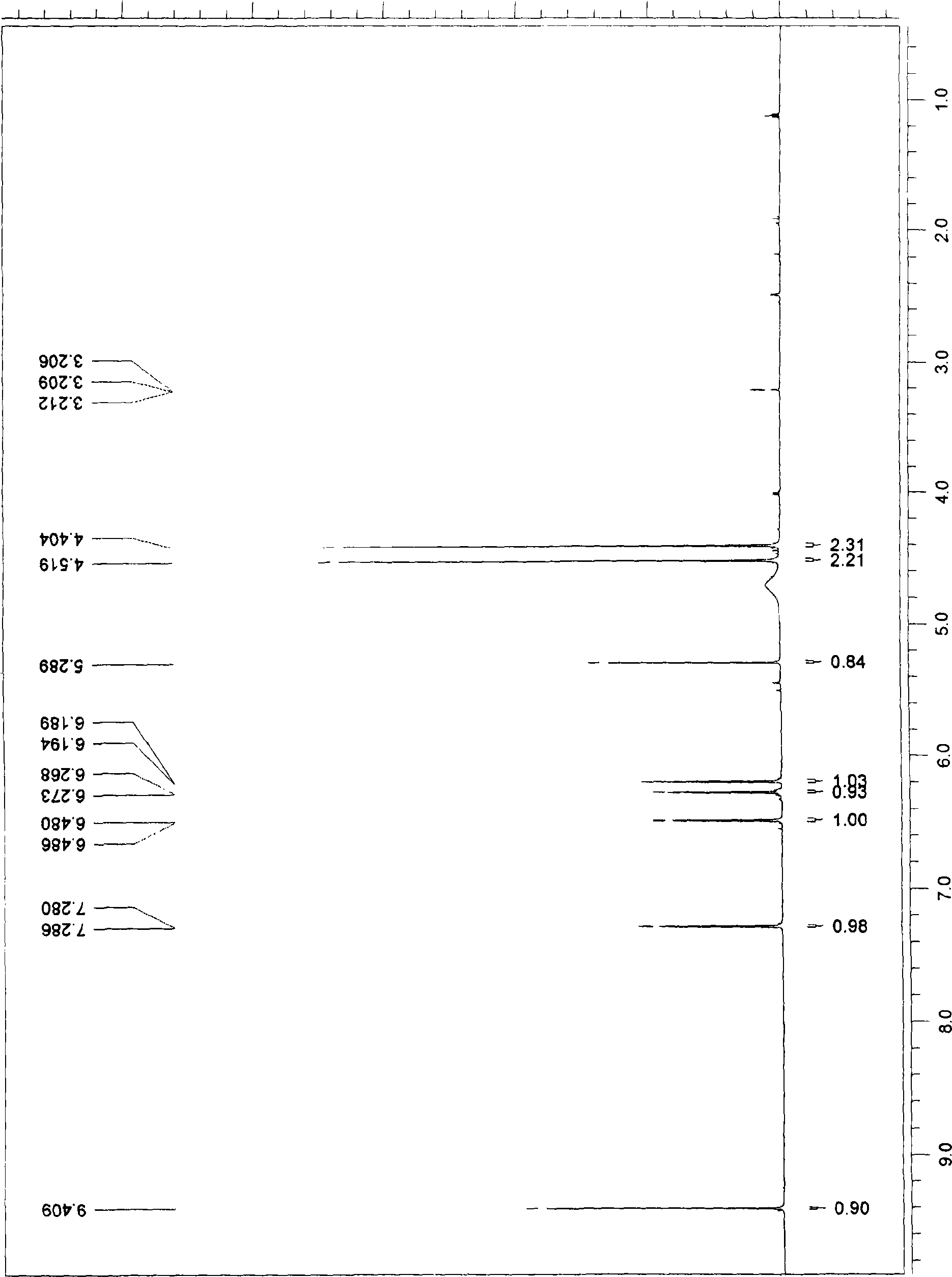
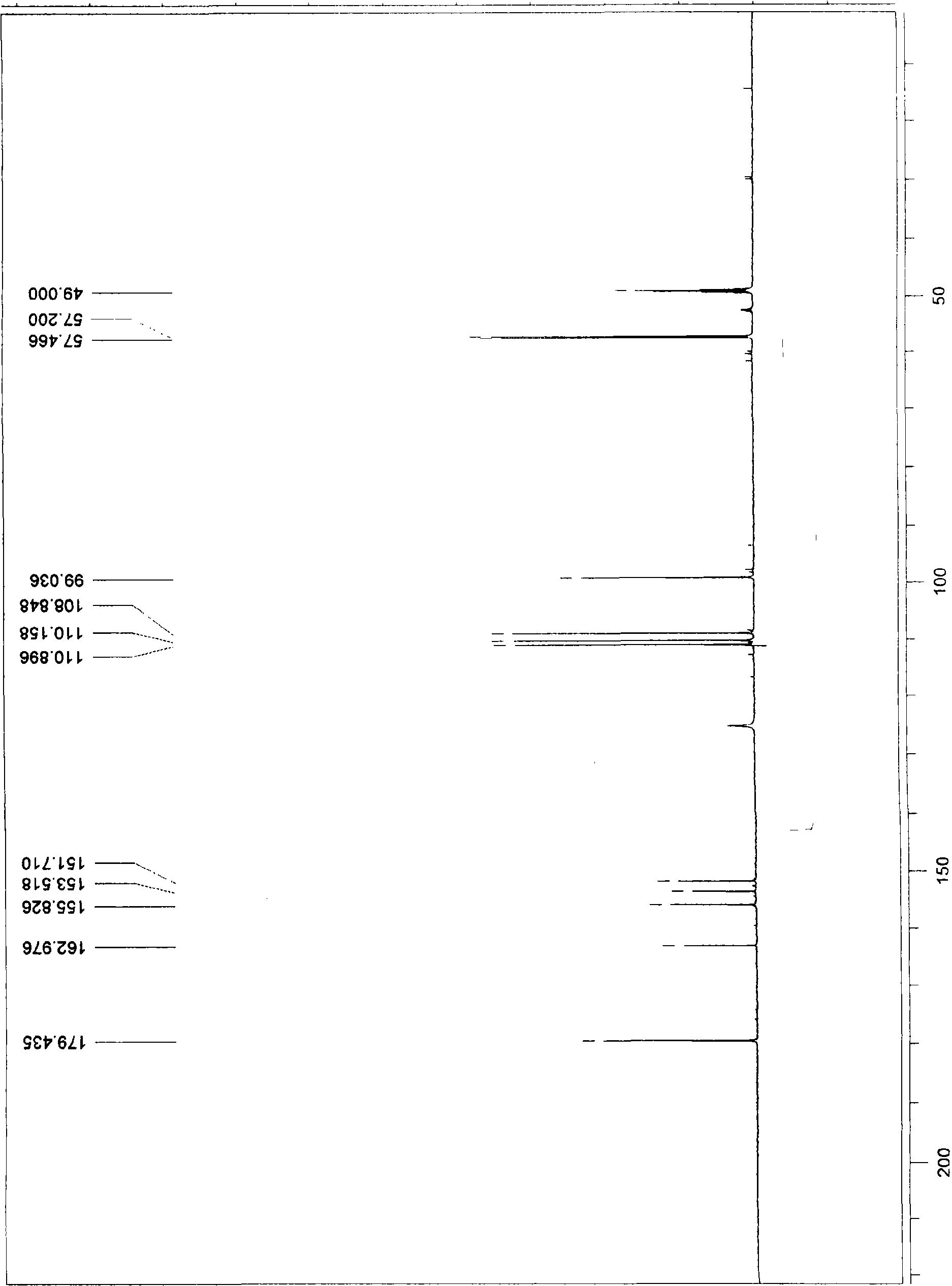5-methylol-furfural-5-furfural-methanol, preparation method thereof and medical application thereof
A technology of hydroxymethylfurfural and methanol, which is applied in the application field of preparing anti-aging drugs, can solve problems such as not seen, and achieve remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] a. Synthesis
[0072] Dissolve 5-HMF in dimethyl sulfoxide, adjust the pH to 3.0 with p-toluenesulfonic acid, raise the reaction temperature to 115°C under stirring conditions, heat and reflux for 3 hours, after cooling, adjust the pH with 0.1M NaOH lye was 7.0, filtered, and the filtrate was concentrated to dryness to obtain the crude product of 5-hydroxymethylfurfural acetal-5-furfural methanol;
[0073] b.Purification
[0074] The crude 5-hydroxymethylfurfural acetal-5-furfural methanol was subjected to silica gel column chromatography and repeated recrystallization from methanol and acetone to obtain pure 5-hydroxymethylfurfural acetal-5-furfural methanol with a yield of 60%.
Embodiment 2
[0076] a.Synthesis method
[0077] Dissolve 5-HMF in dimethyl sulfoxide, adjust the pH to 3.5 with p-toluenesulfonic acid, raise the reaction temperature to 125°C under stirring and heat to reflux for 3 hours, after cooling, use 0.1M NaOH lye to adjust the pH to 7.0, filter, and concentrate the filtrate to dryness to obtain the crude product of 5-hydroxymethylfurfural acetal-5-furfural methanol;
[0078] b.Purification
[0079] Crude 5-hydroxymethylfurfural acetal-5-furfural methanol was subjected to silica gel column chromatography and repeated recrystallization from methanol and acetone to obtain pure 5-hydroxymethylfurfural acetal-5-furfural methanol with a yield of 65%.
Embodiment 3
[0081] a. Synthesis
[0082] Dissolve 5-HMF in dimethyl sulfoxide, add concentrated sulfuric acid dropwise to adjust the pH to 4.0, raise the reaction temperature to 120°C under stirring and heat to reflux for 3 hours, after cooling, adjust the pH to 7.0 with 0.1M NaOH lye , filtered, and the filtrate was concentrated to dryness to obtain the crude product of 5-hydroxymethylfurfural acetal-5-furfural methanol;
[0083] b.Purification
[0084] Crude 5-hydroxymethylfurfural acetal-5-furfural methanol was subjected to silica gel column chromatography and repeated recrystallization from methanol and acetone to obtain pure 5-hydroxymethylfurfural acetal-5-furfural methanol with a yield of 70%.
[0085] The acid used to adjust acidity in the present invention can be concentrated sulfuric acid.
[0086] In the present invention, lye adopts Na 2 CO 3 , KOH solution.
[0087] The embodiment 1 of preparation medicament:
[0088] 5-Hydroxymethylfurfural acetal-5-furfural methanol 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com