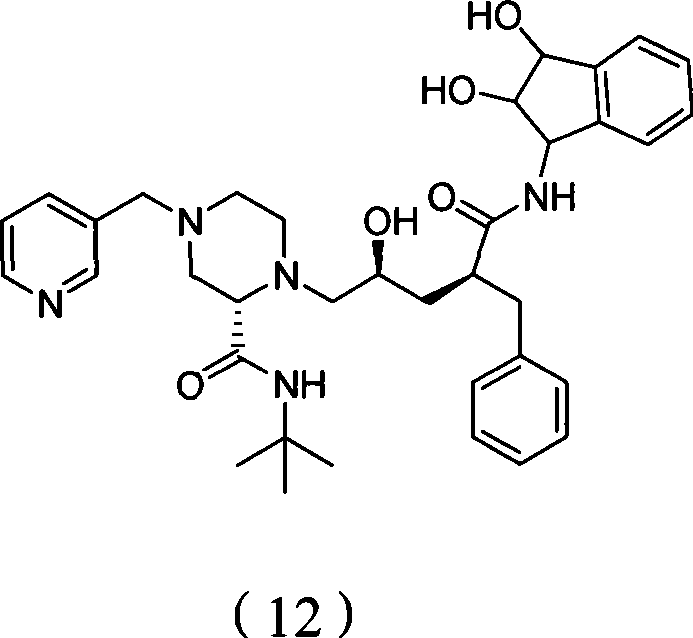
Hydroxylated indinavir, preparation method of hydroxylated indinavir, and application of hydroxylated indinavir in preparation of antimalarials
A hydroxyl and preparation process technology, applied to hydroxylated indinavir and its preparation and application in the preparation of antimalarial drugs, can solve problems such as death and malaria incidence, and achieve broad application prospects and good malaria parasite inhibition activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of compound 2
[0037] Add 150mL water and 150mL tert-butanol to a 500mL round bottom flask, 0.03gK 2 OSo 2 (OH) 4 , 0.33g (DHQD) 2 -PHAL, 42.47g K 3 Fe(CN) 6 and 17.82g K 2 CO 3 , the mixed system was fully stirred at room temperature, and then 4.09 g of methanesulfonamide was added, and the reaction system was stirred at 0° C., and salt precipitated out in the system. During stirring, 5.0 g of indene was slowly added dropwise, and the reaction system was stirred at 0°C for 12 hours. TLC (petroleum ether:ethyl acetate=10:4, v / v) showed that the raw material (indene) disappeared. Add 6g Na to the system 2 SO 3 and stirred at room temperature for 30 minutes, added 150 mL of ethyl acetate, separated the organic phase, extracted three times with ethyl acetate for the aqueous phase, washed the combined organic phase with 50 mL of 2.0N KOH solution, dried over anhydrous magnesium sulfate, and evaporated solvent to obtain 25.73 g of a white powdery solid w...
Embodiment 2
[0047] Synthesis of Compound 8
[0048] In a 250mL round bottom flask, add 4.5g indinavir, 120mL methanol, place the flask in an ice bath and stir, slowly add a preconfigured NaOH aqueous solution (6.6g NaOH is dissolved in 40mL water) dropwise through a constant pressure dropping funnel, During the dropwise addition, the solution gradually became white and turbid. After the dropwise addition, the ice bath was removed, heated to 40°C in a water bath and stirred for 24 hours. TLC (dichloromethane:methanol=10:1, v / v) showed that the raw materials disappeared. Under ice bath and vigorous stirring, slowly add 10% methanolic HCl solution dropwise until the pH of the system is 8, rotary evaporate and add anhydrous methanol several times to take out the moisture in the mixture, and dissolve the obtained solid with 80 mL of ethyl acetate and filter, The filter cake was washed three times with ethyl acetate, and the combined solution was dried over anhydrous sodium sulfate and rotary e...
Embodiment 3
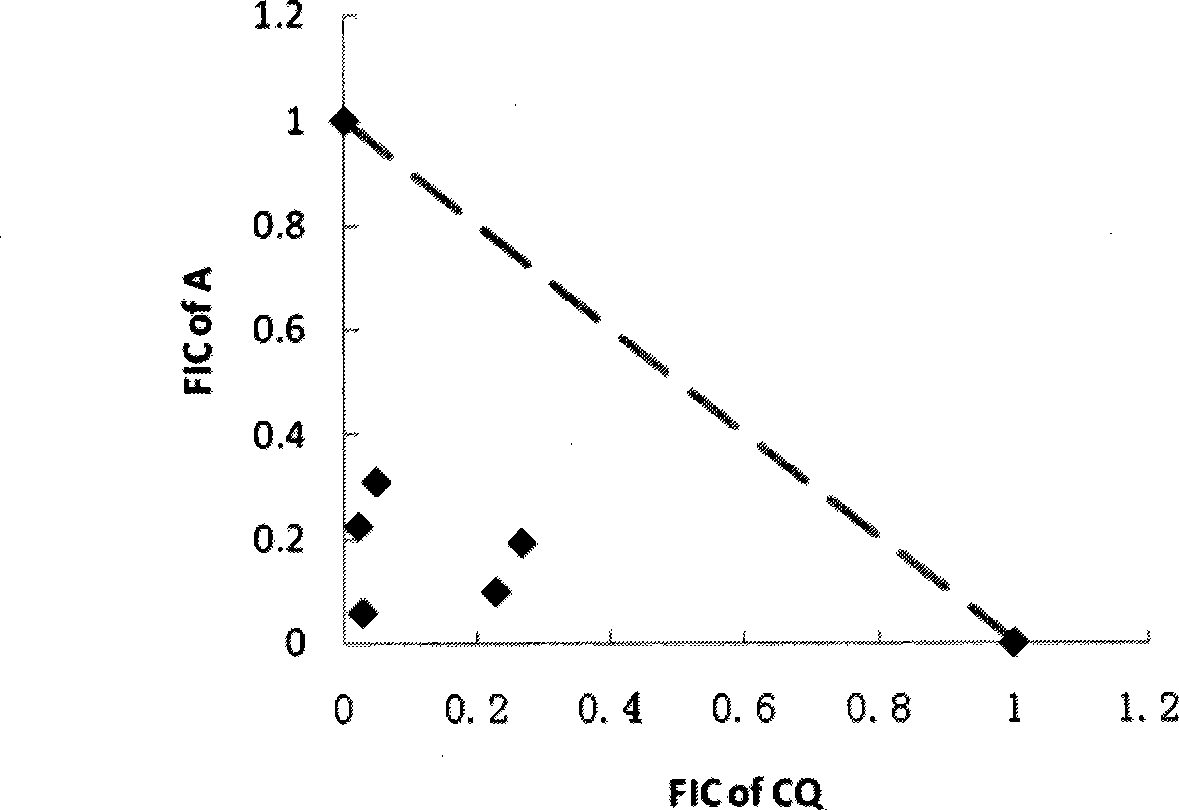
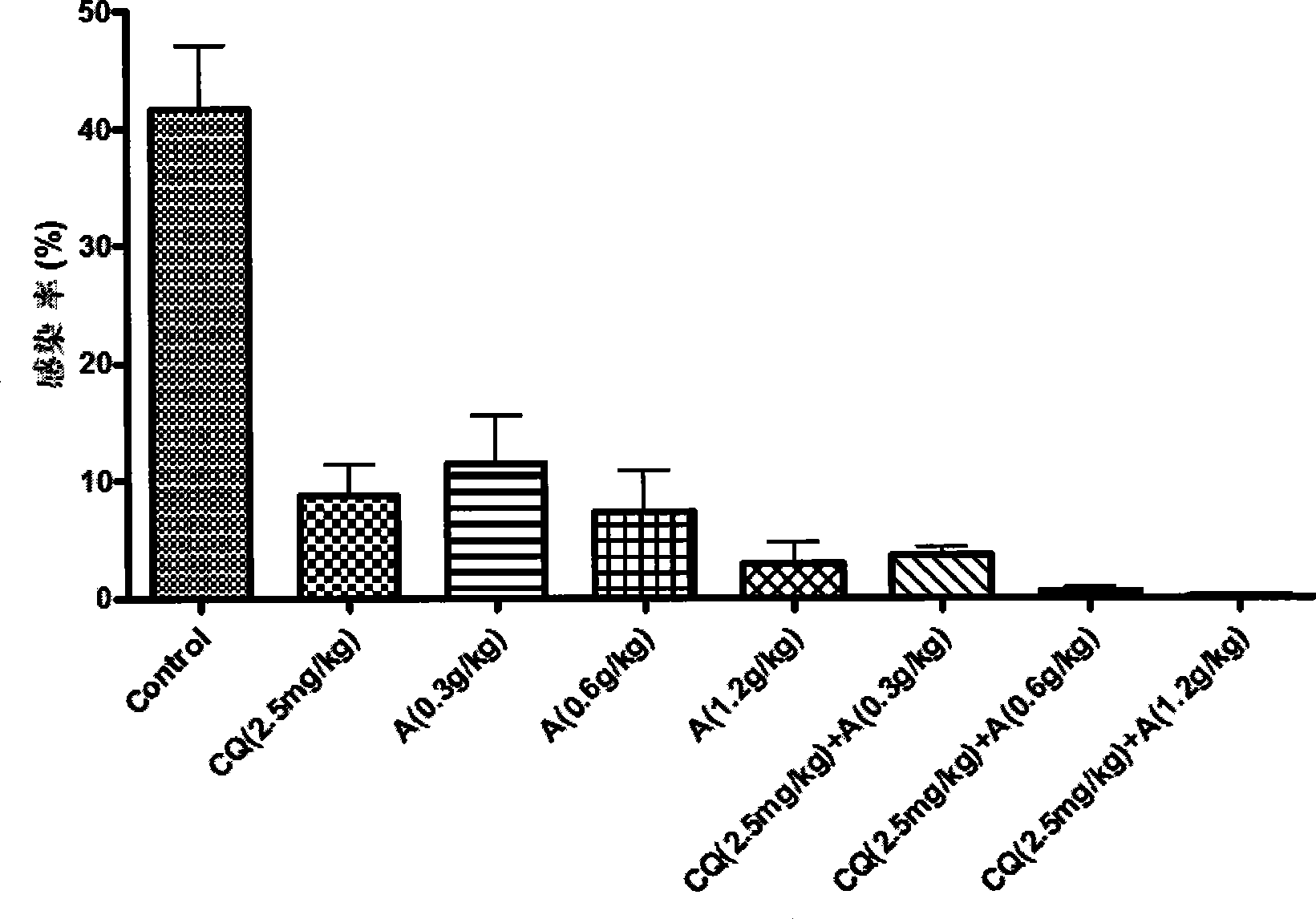
[0057] Example 3 Evaluation of the antimalarial effect of hydroxylated indinavir in vitro
[0058] Taking the two stereoisomers of hydroxylated indinavir (hydroxylated indinavir A and hydroxylated indinavir B) as an example, hydroxylated indinavir is effective against human Plasmodium falciparum (P.falciparum) Both the sensitive strain 3D7 and the multidrug-resistant strain Dd2 had strong inhibitory effects.
[0059]
[0060] Hydroxylated Indinavir A Hydroxylated Indinavir B
[0061] The human P. falciparum (P. falciparum) sensitive strain 3D7 and the multi-drug resistant strain Dd2 were cultured in vitro with RMPI1640 medium + 10% human serum, and the hematocrit was about 5%. Synchronize Plasmodium parasites with 5% D-sorbitol, plate at their ring body stage, with an initial infection rate of 1% and a hematocrit of 5%. The drug is dissolved in DMSO to prepare a mother solution, and then diluted with a culture medium. When the activity is detected, the concentration gradi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com