Preparation method of ceftezole and midbody thereof
A technology for ceftazole and intermediates, which is applied in the field of preparation of cephalosporin compounds, can solve the problems of poor product color and purity, low reaction yield and the like, and achieves short reaction time, low cost, and improved yield and purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
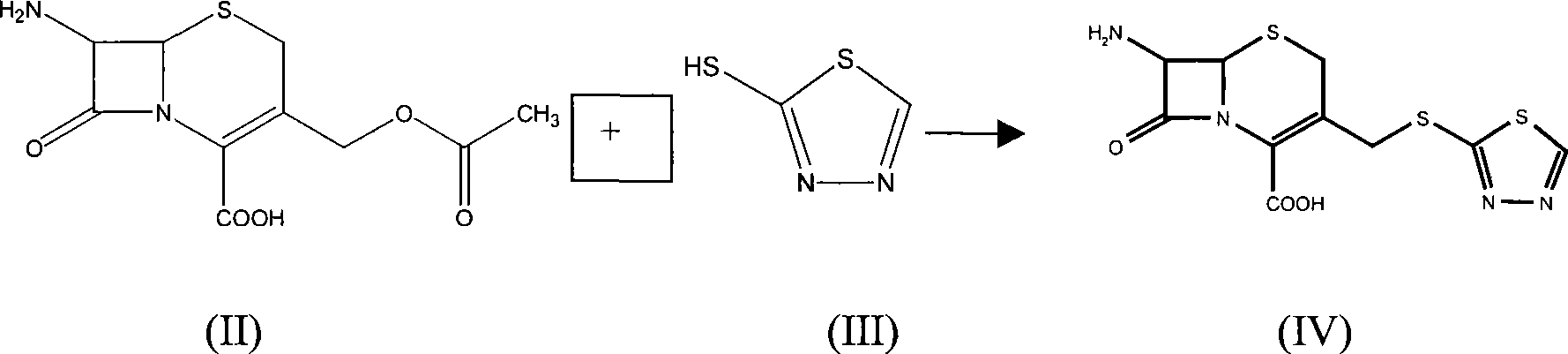
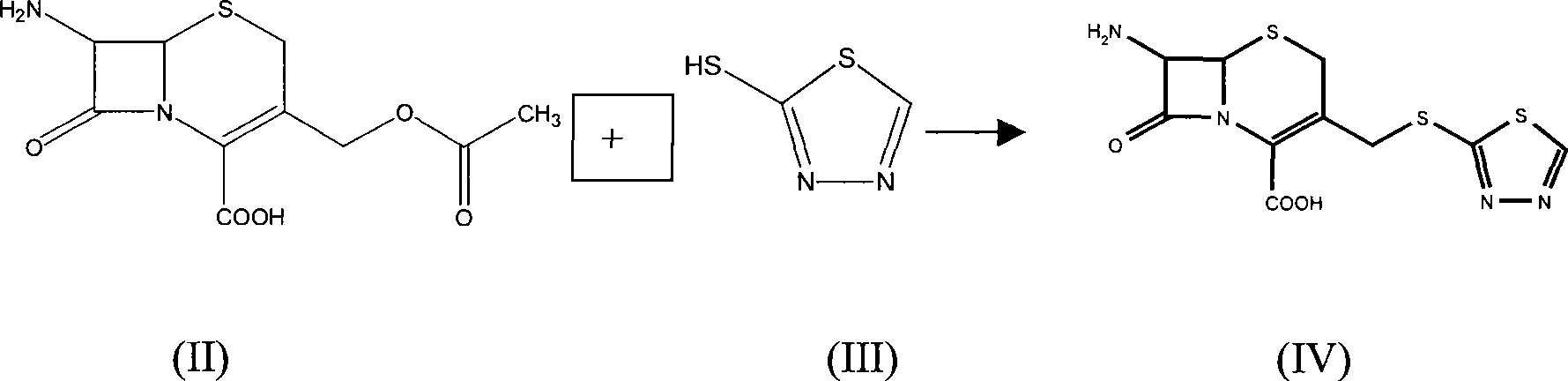
[0023] 5g (16mmol) of 7-ACA was dissolved in 120ml of water, and 3ml of triethylamine was added dropwise to dissolve to prepare a triethylamine solution of 7-ACA for future use. Put 5-mercapto-1,3,4-thiadiazole (4.5g, 38mmol) into a 300ml reaction flask, add 4g of boric acid, 300ml of water, stir to dissolve, heat to 65-70°C, add dropwise 7 - The triethylamine solution of ACA, continue to stir for about 25 minutes after dropping, cool down to 40-50°C, then cool down to 0-5°C, stir for 0.5h, filter, and dry to obtain 5g of solid. It was detected by standard TLC as 7-amino-3-(1,3,4-thiadiazol-5-yl)thiomethyl-2-cephem-2-carboxylic acid.
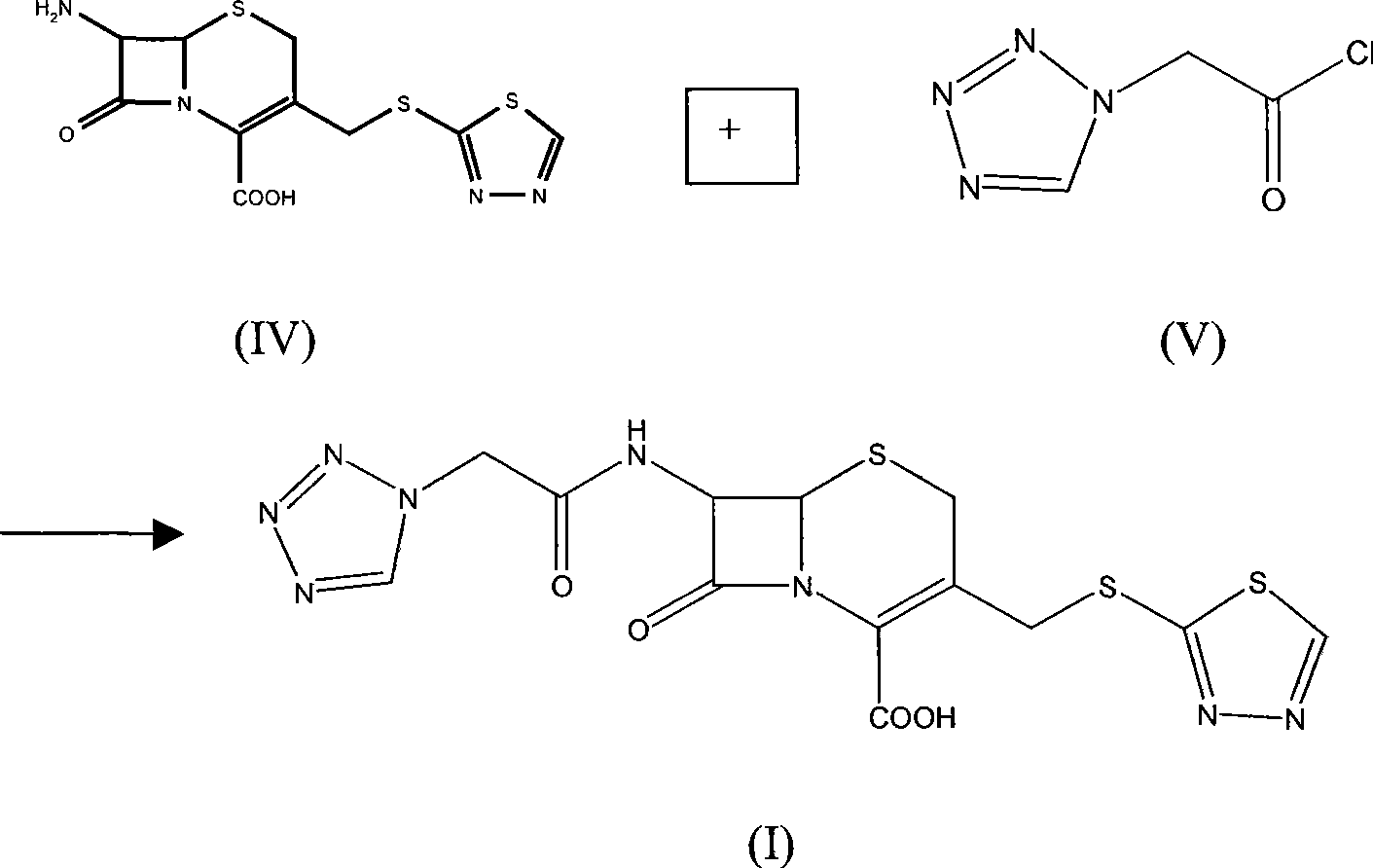
[0024] Put 5g of 7-amino-3-(1,3,4-thiadiazol-5-yl)thiomethyl-2-cephem-2-carboxylic acid into the reaction flask, add 350ml of water, 300ml of acetone, stir, and then Use triethylamine to adjust the pH to between 7.0 and 7.5, cool down to 0-5°C, slowly add a mixed solution of 1H-tetrazolium-1-acetyl chloride (6.5 g) and acetone (100 ml) dropwise...
Embodiment 2
[0026] 5g (16mmol) of 7-ACA was dissolved in 120ml of water, and 3ml of triethylamine was added dropwise to dissolve to prepare a triethylamine solution of 7-ACA for future use. Put 5-mercapto-1,3,4-thiadiazole (4.5g, 38mmol) into a 300ml reaction flask, add 4g of boric acid, 300ml of water, stir to dissolve, heat to 65-70°C, add dropwise 7 - The triethylamine solution of ACA, continue to stir for about 25 minutes after dripping, cool down to 40-50°C, then cool down to 0-5°C, stir for 0.5h, add 250 ml of acetone to the aqueous solution, stir, and at the same time use triethylamine Adjust the pH to between 7.0 and 7.5, lower the temperature to 0-5°C, slowly add a mixed solution of 1H-tetrazolium-1-acetyl chloride (6.5 g) and acetone (100 ml) dropwise, and maintain the pH at Between 5.6 and 7.0, react for 0.5 hours after dropping, concentrate the acetone, adjust the pH of the aqueous phase to less than 2.5 with 3mol / L hydrochloric acid, and obtain an off-white solid, which is de...
Embodiment 3
[0028] Dissolve 8 g of sodium 7-ACA in 150 ml of water to prepare a sodium 7-ACA solution for later use. Put 5g of 5-mercapto-1,3,4-thiadiazole into a 300ml reaction bottle, add 5g of boric acid and 300ml of water, stir to dissolve, heat to 65-70°C, add 7-ACA sodium solution dropwise while stirring After dropping, continue to stir for about 25 minutes, cool down to 40-50°C, then cool down to 0-5°C, stir for 0.5h, add 250 ml of acetone to the aqueous solution, stir, and adjust the pH to 7.0-7.5 with triethylamine at the same time During this period, the temperature was lowered to 0-5°C, and a mixed solution of 1H-tetrazolium-1-acetyl chloride (6.5 g) and acetone (100 ml) was slowly added dropwise, and the pH was maintained between 5.6 and 7.0 with saturated sodium carbonate solution, and the After completing the reaction for 0.5 hour, concentrate the acetone, and adjust the pH of the aqueous phase to less than 2.5 with 3 mol / L hydrochloric acid to obtain an off-white solid, whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com