Method for testing electrochemical performance of polyaniline synthesized by chemical method
A test method and technology of polyaniline, applied in the direction of electrochemical variables of materials, can solve the problem that the electrochemical properties of polyaniline are difficult to effectively characterize, and achieve the effect of good electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
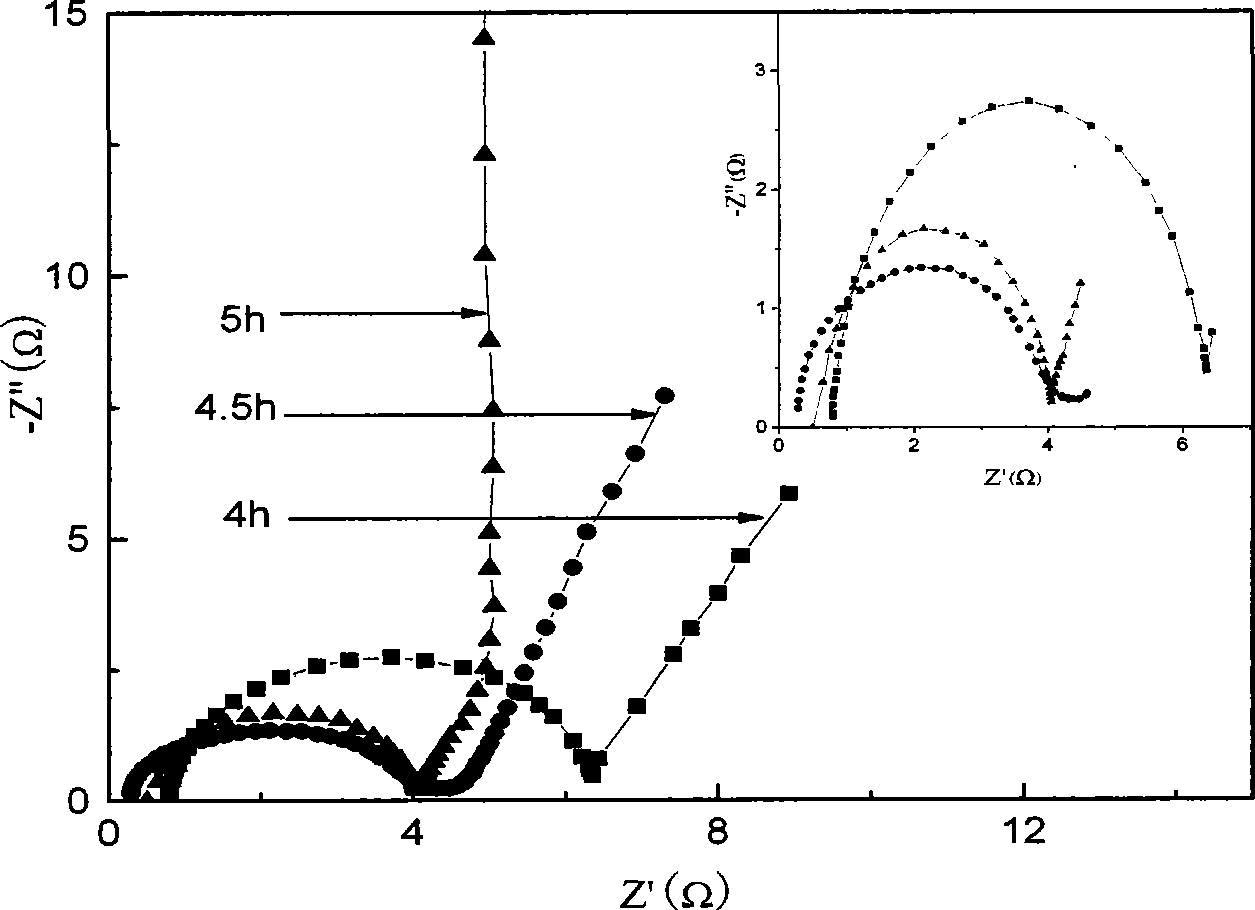
[0018] Weigh sodium dodecylbenzenesulfonate into a three-necked flask, add hydrochloric acid and stir until it is completely dissolved. Add carbon paper into the system to make it fully wet, add the configured initiator ammonium persulfate solution dropwise, and react for 4 to 5 hours. After the reaction, take out the carbon paper, and use the polyaniline-loaded carbon paper as an electrode to carry out polymerization. Electrochemical performance test of aniline.
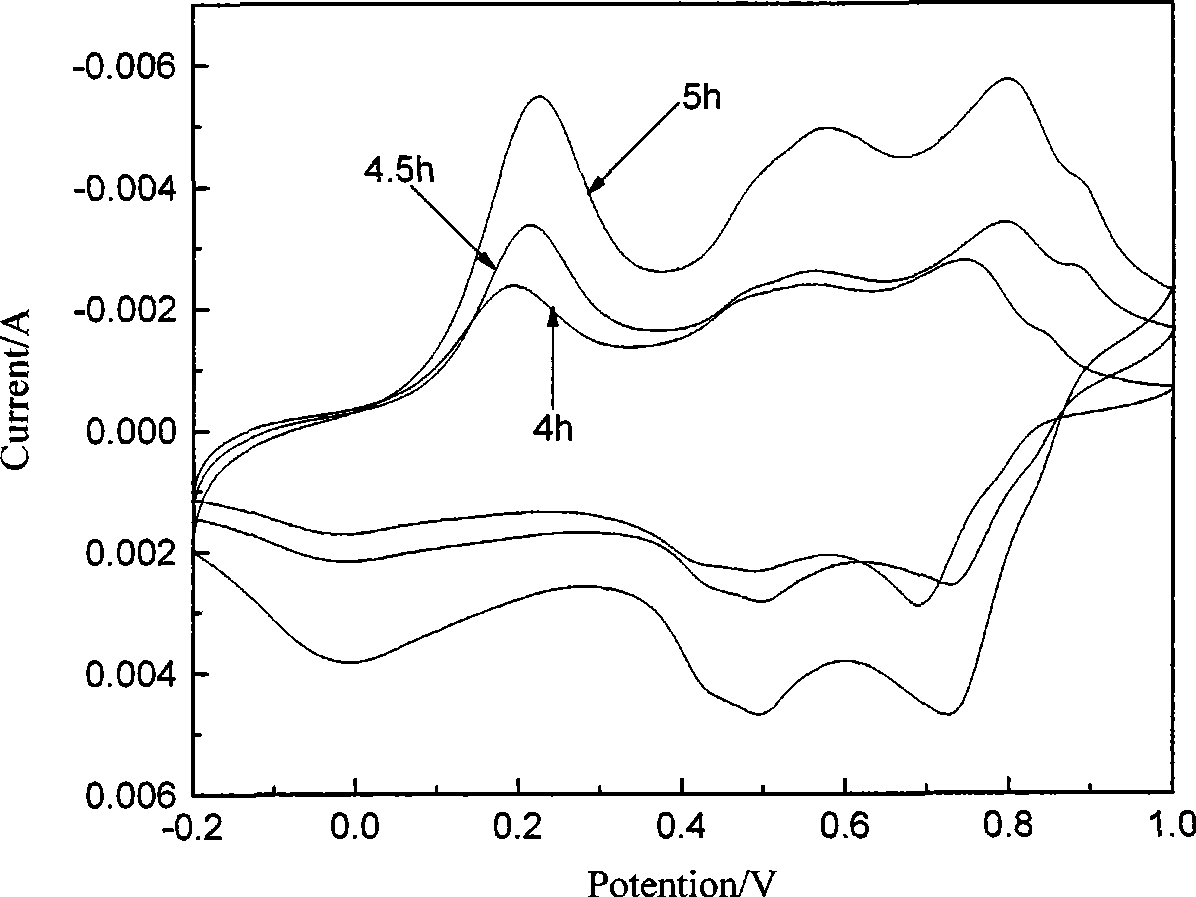
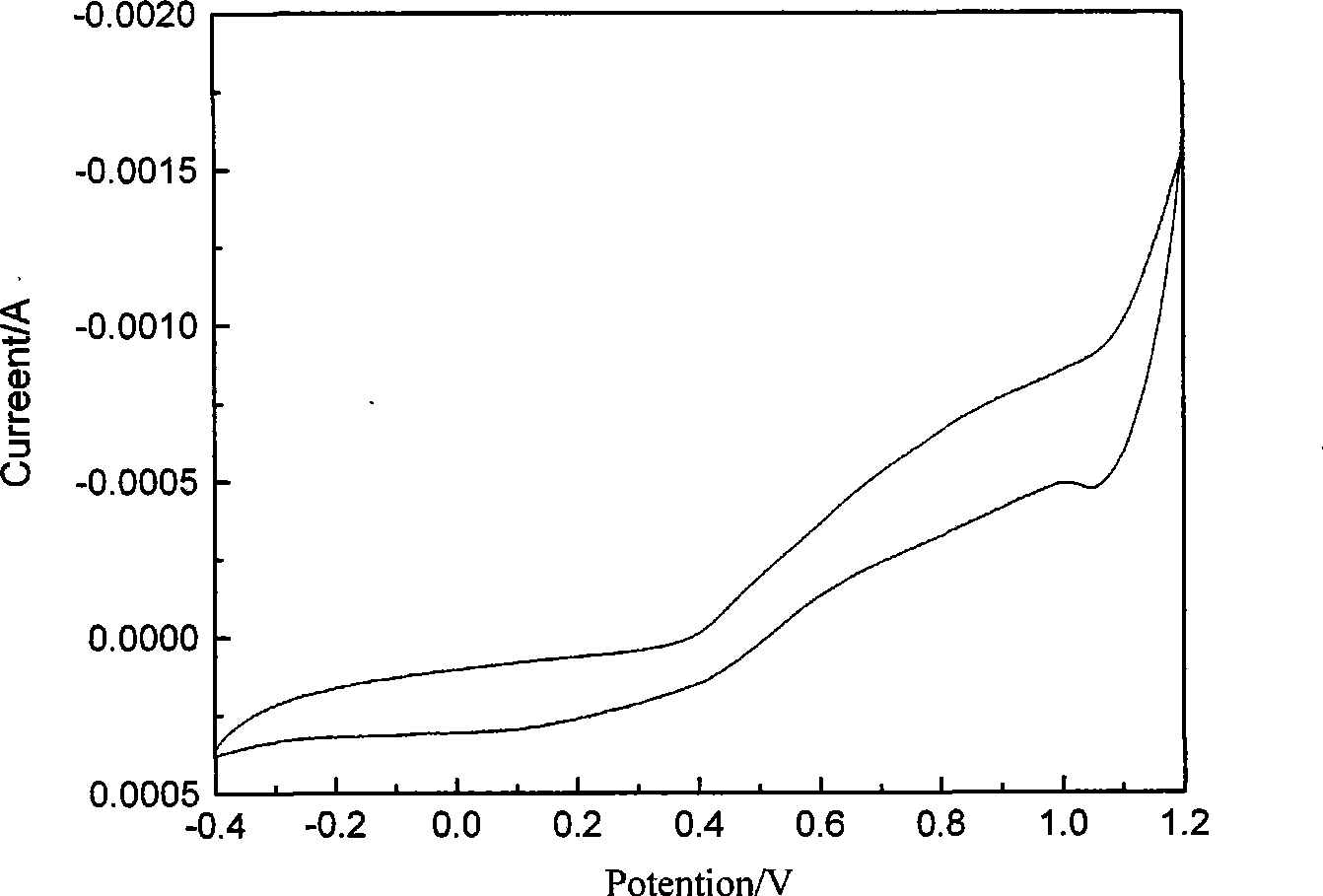
[0019] Depend on figure 1 It can be seen that there are three pairs of relatively obvious redox peaks around 0.2, 0.6 and 0.8V, which is consistent with Itava K et al. -382) The same as reported in the literature, that is, the peak at 0.2V corresponds to the process of oxidation of aniline to positive ion groups, the peak at 0.8V corresponds to the process of further oxidation to its quinoid compound, and the middle peak corresponds to the process of polyaniline The degradation product benzoquinone is also consist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com