Real-time fluorescence PCR reagent kit for human metapneumovirus
A metapneumovirus, real-time fluorescence technology, applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc., can solve problems affecting virus research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
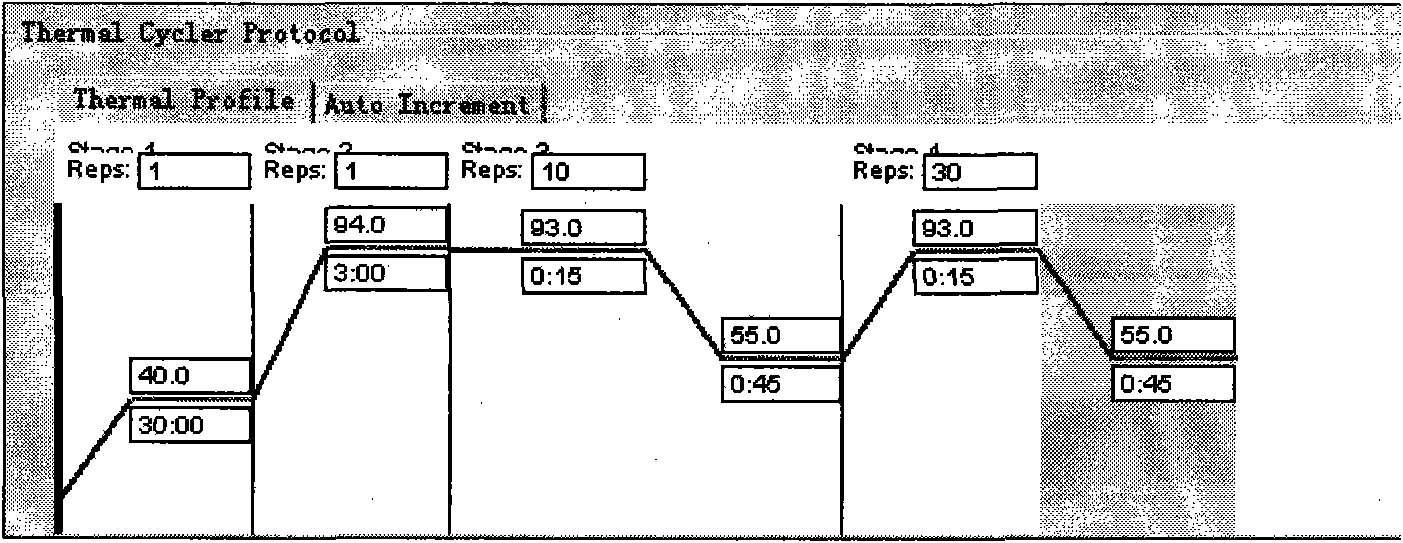
[0074] Embodiment 1: One-step kit detection method of human metapneumovirus
[0075] (1) Specimen collection, transportation and storage
[0076] Specimens were collected by clinicians according to the actual situation. Common specimen collection methods include the following: immerse the swab in 4-5ml of sampling liquid, seal it and send it for inspection; sputum samples should be sent for inspection immediately after collection, or stored at -20°C for testing. Testable specimens include sputum, throat swabs, and bronchoalveolar lavage fluid. The collection methods are as follows: ① Sputum: expectorate naturally or induce sputum suction (such as positive oxygen pressure method, disposable baby suction tube method, and nebulized steam inhalation method), suck sputum, and seal it for inspection; ② nasopharyngeal swab Son: Wipe bilateral pharyngeal tonsils and posterior pharyngeal wall with a swab, avoid touching the tongue, or insert the swab into the nasal cavity along the d...
Embodiment 2
[0088] Embodiment 2: two-step kit detection method of human metapneumovirus
[0089] (1) Specimen collection, transportation and preservation
[0090] Same as (1) in Example 1
[0091] (2) Nucleic acid extraction
[0092] Add 10 μl of well-mixed RNA extraction solution A (DEPC.H 2 O diluted, and autoclaved after 10% SiO 2 ; RNA extraction solution A is easy to precipitate, and it needs to be mixed with a pipette repeatedly before sampling). Then add 200 μl of RNA extraction solution B (guanidine isothiocyanate base solution) and mix thoroughly. After standing at room temperature for 5 minutes, centrifuge at 8000 revolutions per minute (rpm) for 1 minute, discard the supernatant; repeatedly add 200 μl of RNA extraction solution B to mix well, centrifuge at 8000 revolutions per minute (rpm) for 1 minute, discard the supernatant; % pre-cooled ethanol (prepared with DEPC water) to wash the precipitate twice (take 400 μl of 75% ethanol to wash the precipitate, shake and mix we...
Embodiment 3
[0102] Example 3: Preparation and use of quantitative reference substance and quality control substance in human metapneumovirus nucleic acid quantitative detection kit
[0103] The quantitative reference substance in the Human Metapneumovirus Nucleic Acid Quantitative Detection Kit is in vitro transcribed RNA, which is used to prepare a standard curve for accurate quantification of the samples to be tested, and can be directly used for RT-PCR detection; quality control substances include positive quality control substances and negative Quality control product, used for quality control in clinical trials, the operation method is the same as that of samples to be tested.
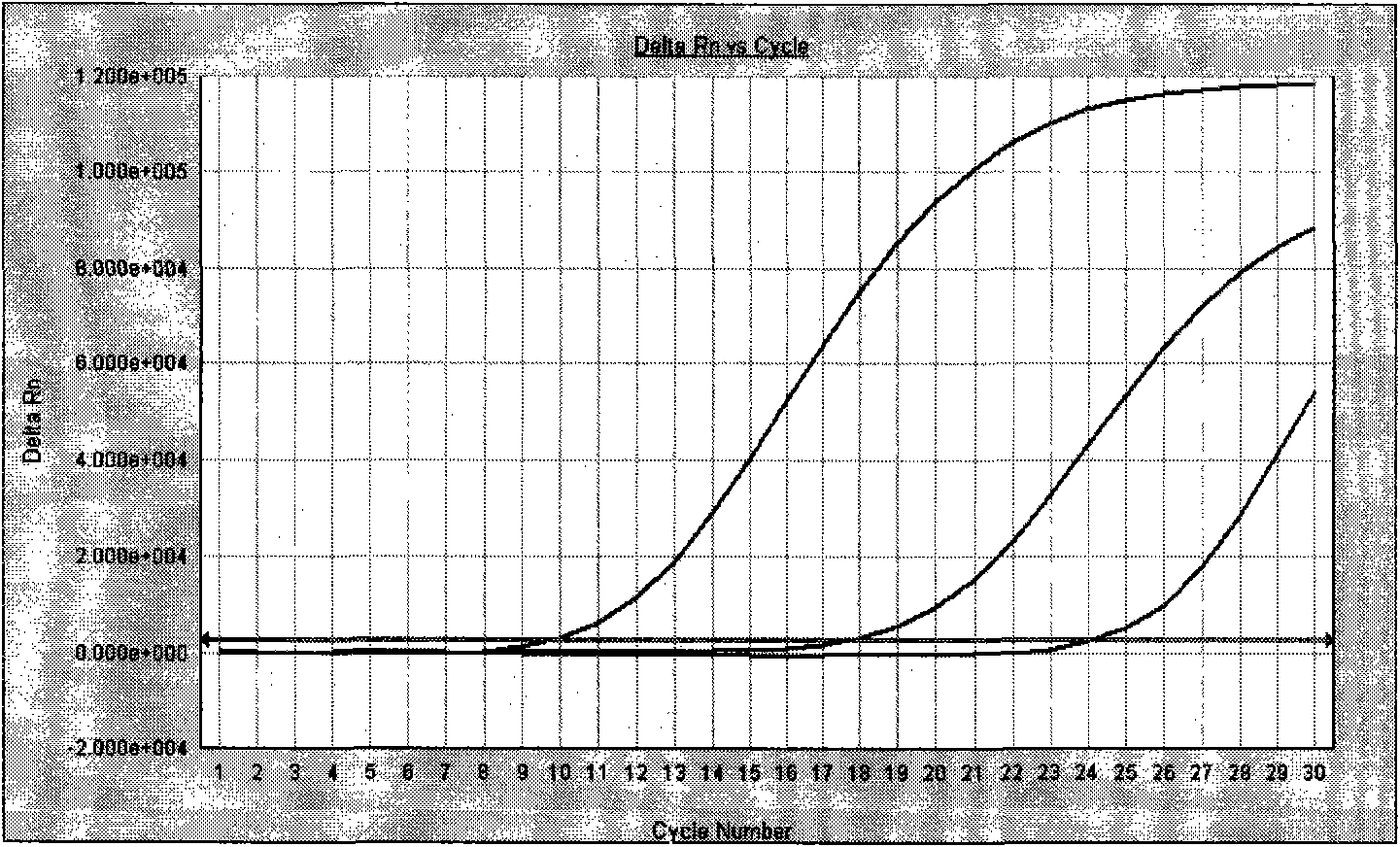
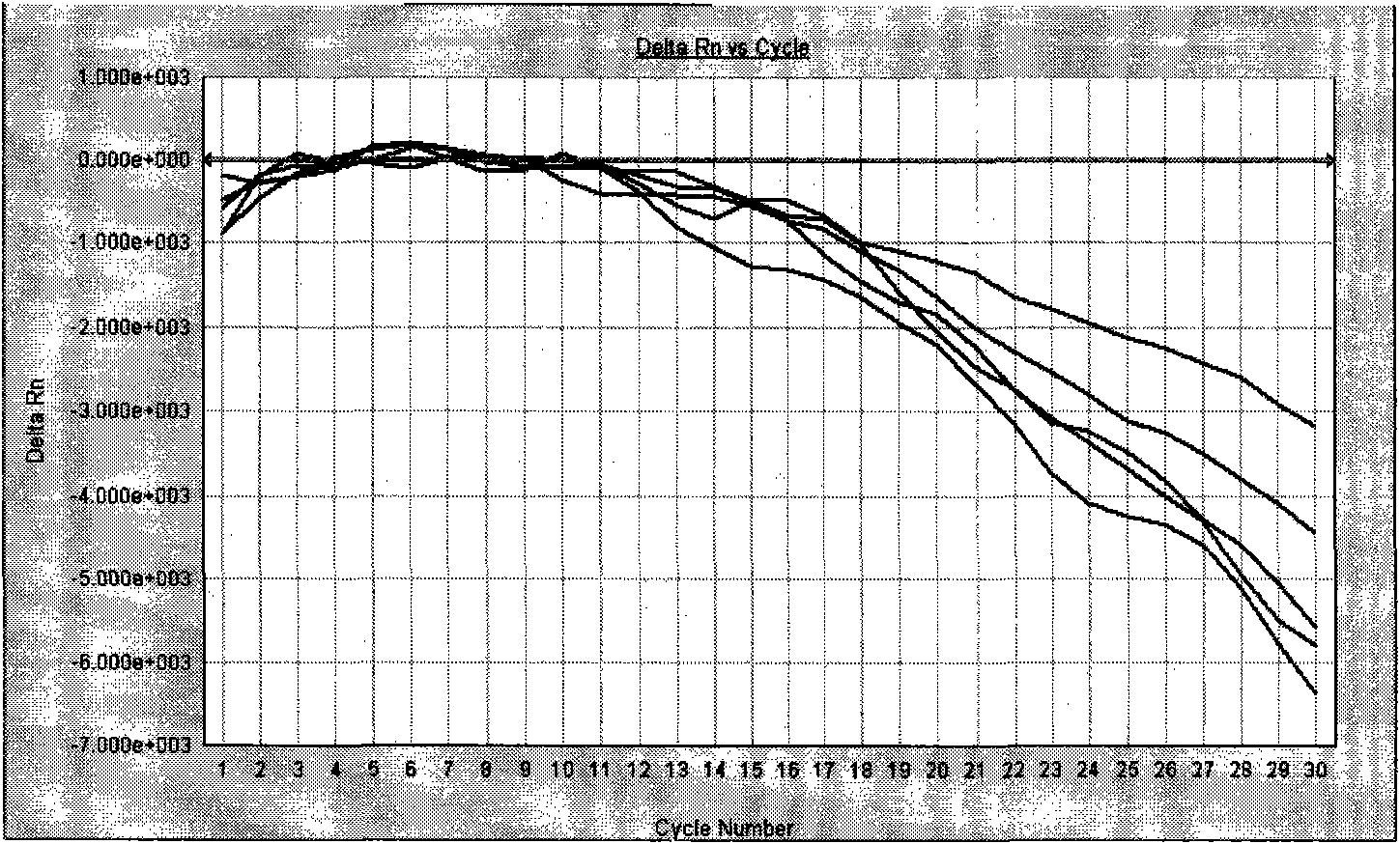
[0104] After one-step or two-step amplification, save the detection data file. Adjust the analysis parameters according to the analyzed image to optimize the standard curve graph under the standard curve (Std curve) window (that is, the correlation (correlation) value Figure 9 , and the information about the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com