Human recombinant amelogenin and preparation method thereof
A technology of amelogenin and fusion protein, which is applied in the protein field to achieve the effect of promoting expression, increasing solubility, and increasing protein stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
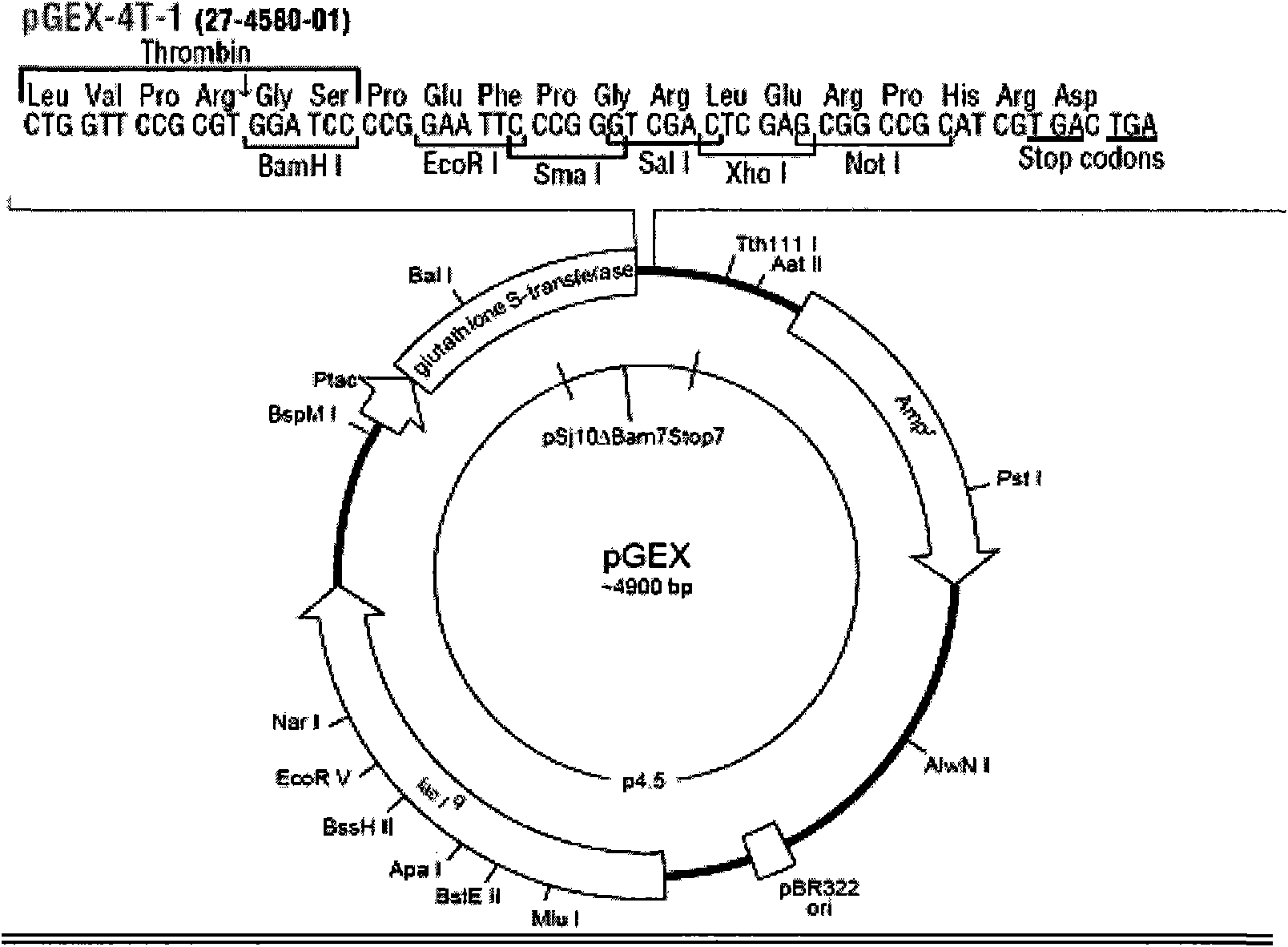
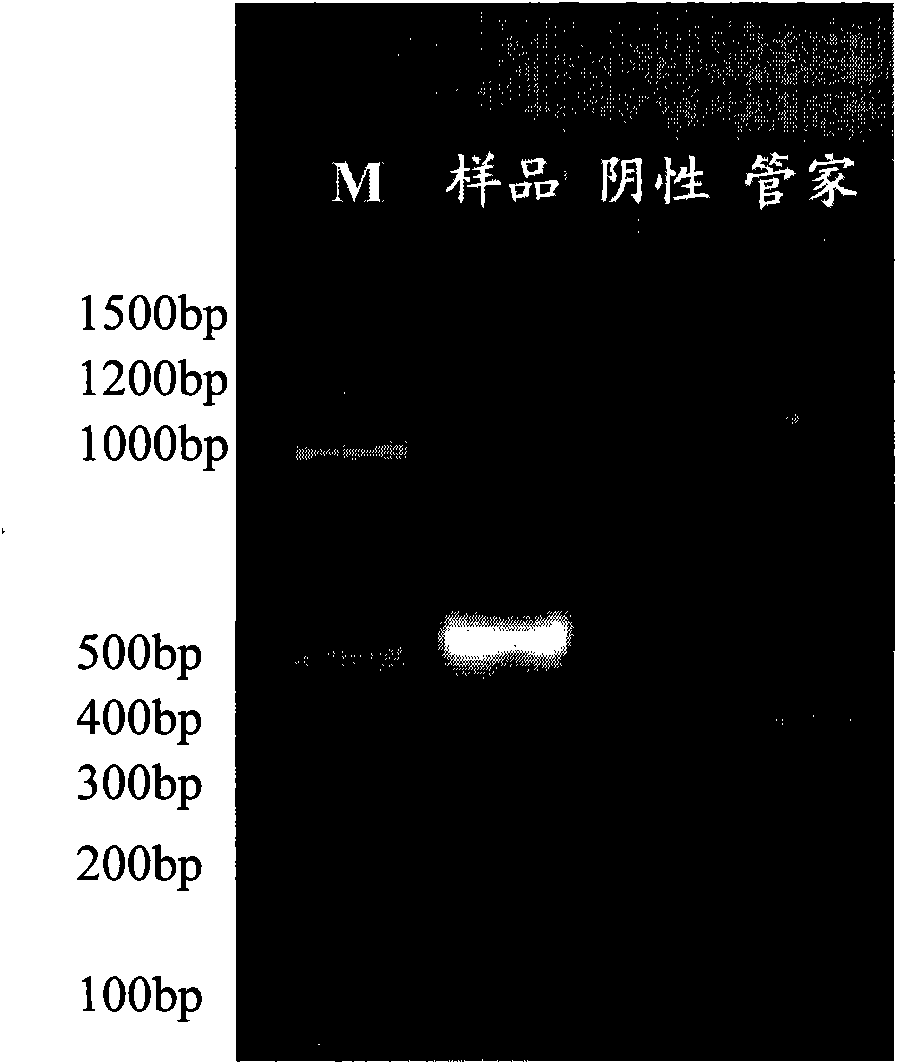
[0038] A kind of recombinant human amelogenin preparation
[0039] I. Materials and methods for cloning human amelogenin mature peptide gene hAm encoded by X chromosome
[0040] 1. Main experimental materials
[0041] 1. Human tooth germ tissue: The laboratory of the Ninth People's Hospital Affiliated to Shanghai Second Medical University.
[0042] 2. Main reagents and solutions
[0043] (1) Total RNA Extraction Kit (Baicai Biotechnology Co., Ltd.)
[0044] (2) c DNA First Strand Synthesis Kit (Baicai Biotechnology Co., Ltd.)
[0045] (3) Small amount of glue recovery kit (Tiangen Company)
[0046] (4) PCR reagents: Taq DNA polymerase, 10× amplification buffer, MgCL 2 , 10mmol / L 4×dNTP (Pro omeg company)
[0047] (5) DEPC (Boca Biotechnology Co., Ltd.)
[0048] (6) DNA markers
[0049] (7) 6× agarose electrophoresis sample solution: consists of the following components: 2.5g / L bromophenol blue (adjusted to dark blue pH=8.0 with 0.5mol / L NaOH), 400g / L sucrose, 10mmol / L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com