Norcantharidin esterified derivatives and preparation method thereof
A technology for cantharidin esters and derivatives, which is applied in the field of synthesis of norcantharidin esterified derivatives, can solve the problems of undisclosed secondary products and the like, and achieve the effects of rapid and accurate content and control of the reaction process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Preparation of 7-oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid butyl ester
[0088]
[0089] C 16 H 26 O 5
[0090] 298.38
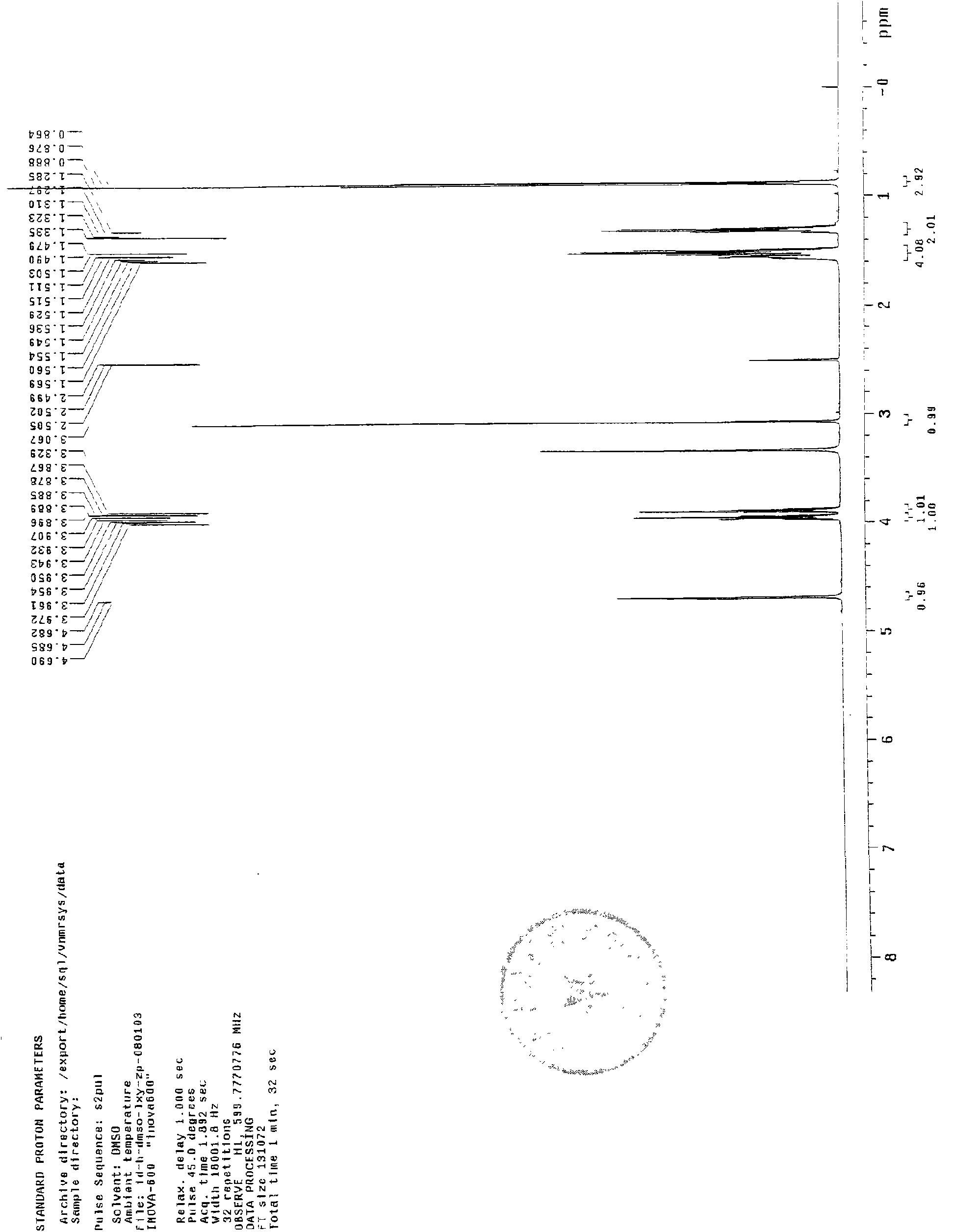
[0091] Add 2g norcantharidin into the reaction flask, then add 6ml excess n-butanol, then add H 2 SO 4 0.1ml mix. Place the reaction flask on a thermostatic magnetic stirrer, stir and heat to 80°C-100°C and reflux for 70 minutes. After the reaction was terminated, an oily liquid was obtained. The product was washed with cold water 3 times×20ml, and a white waxy solid was obtained by suction filtration. The white solid was dissolved by adding preheated n-hexane at 45°C, and crystallized in the refrigerator. The next day, suction filtration gave a white solid crystal 7 -Oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid butyl ester, weighing a total of 2.24g.
[0092] TLC identification
[0093] Developing agent: chloroform: acetone: formic acid = 97: 3: 2
[0094] TLC color development method: iodine cylinder smoked.
[0095] Tests have proved that usi...
Embodiment 2
[0099] Preparation of 7-oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid hexyl ester
[0100]
[0101] C 20 H 34 O 5
[0102] 354.49
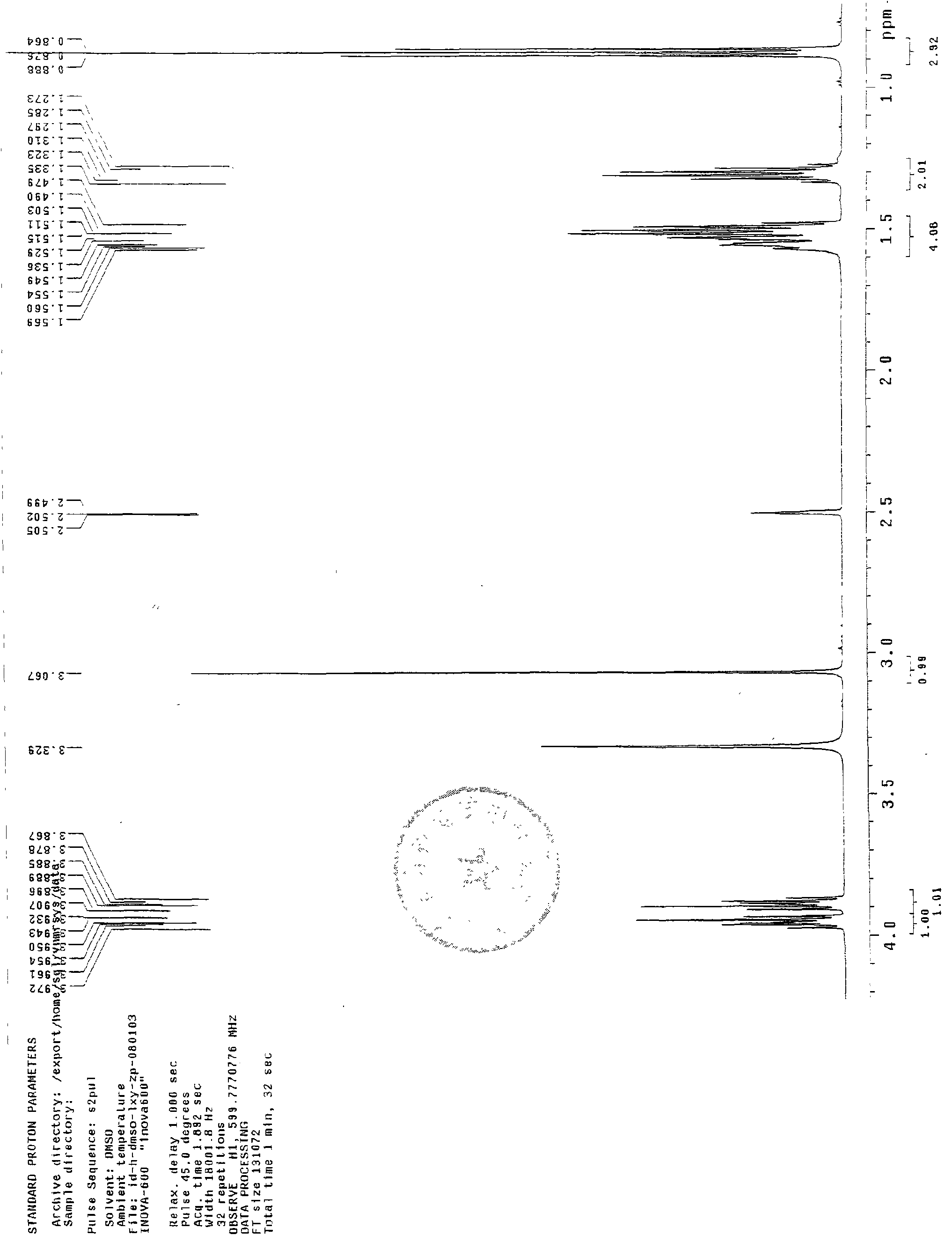
[0103] Synthesis steps: add 7.5ml (0.06mol) excess n-hexanol to the reaction flask, add 3.36g (0.02mol) norcantharidin, and then add H 2 SO 40.12ml mix. Place the reaction flask on a thermostatic magnetic stirrer, stir and heat to 80°C and reflux for 65 min. After the reaction was terminated, an oily liquid was obtained. The product was washed with cold water 3 times×20ml, and a white waxy solid was obtained by suction filtration. The white solid was dissolved by adding preheated n-hexane at 45°C, and crystallized in the refrigerator. The next day, suction filtration gave a white solid crystal 7 -Oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid hexyl ester, weighing a total of 3.56g pure product.
[0104] TLC identification
[0105] Developing agent: chloroform: acetone: formic acid = 97: 3: 2; the rest are the same as in Example 1.
Embodiment 3
[0107] Synthesis of 7-oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid octyl ester
[0108] The reaction formula is:
[0109]
[0110] C 24 H 42 O 5
[0111] 410.59
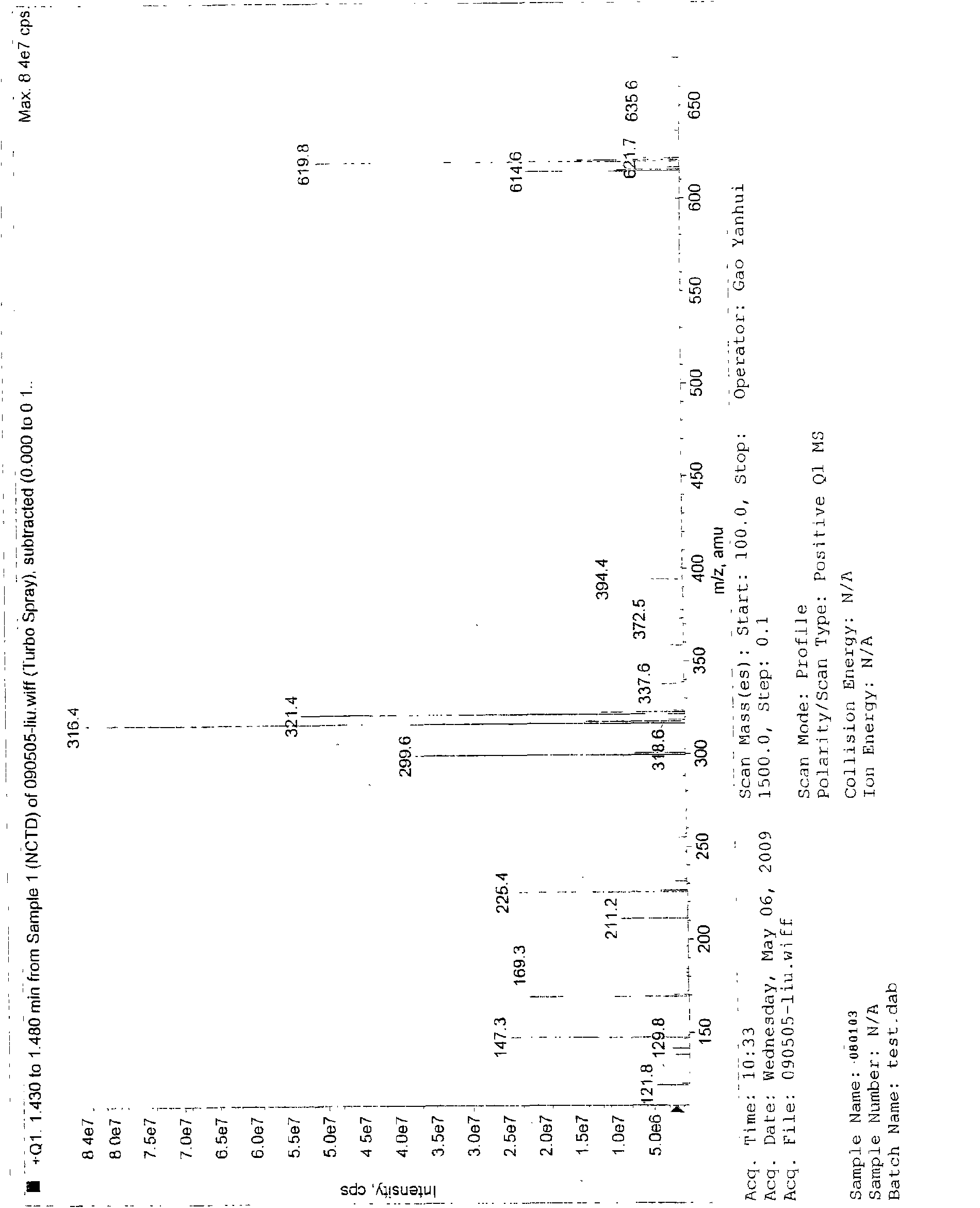
[0112] Add 6ml excess n-octanol to the reaction flask, add 2g norcantharidin, and then add H 2 SO 4 0.06ml mix. Place the reaction flask on a thermostatic magnetic stirrer and stir and heat to 45°C, reflux for 5 hours and terminate the reaction to obtain an oily liquid. The product is washed 3 times with cold water × 20ml, filtered with suction to obtain a white waxy solid, and dissolved in preheated n-hexane at 45°C. The white solid was crystallized in the refrigerator, and filtered with suction after 1-24 hours to obtain 7-oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid octyl ester, and weighed to obtain 2.27g pure product.
[0113] TLC identification
[0114] Developing agent: chloroform: acetone: formic acid = 97: 3: 2
[0115] TLC color development method: iodine cylinder smoked.
[0116] Tests have proved that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com