Process for preparing phosphate concentrate and by-products calcium carbonate and magnesium oxide from mid and low-grade phosphate rocks
A low-grade, phosphorus concentrate technology, applied in the direction of magnesium oxide, calcium carbonate/strontium/barium, phosphate material treatment, etc., can solve the problems of land occupation and processing difficulties, and achieve the reduction of magnesium content, improvement of purity, and good economy benefit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
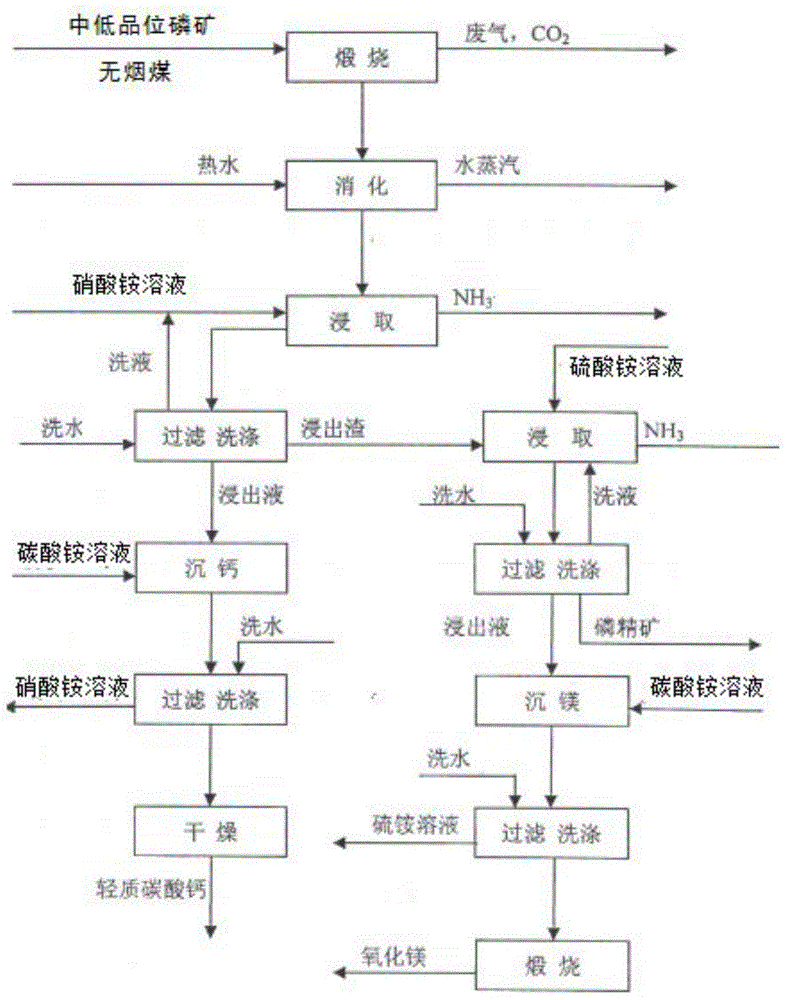
[0042] A process for preparing phosphate concentrate by-product calcium carbonate and magnesium oxide from medium and low-grade phosphate rock, the process flow chart is as follows figure 1 shown, including the following steps:
[0043] (1) Calcining 100g of low- and medium-grade phosphate rock at 900-1100°C for 1 hour (the factory calcination process is to mix low- and medium-grade phosphate rock with anthracite for calcination) to obtain calcined slag and carbon dioxide; Dolomite (CaCO 3 · MgCO 3 ), calcite (CaCO 3 ) and fluorapatite (Ca 5 F(PO 4 ) 3 ), where dolomite and calcite begin to decompose at high temperatures above 750°C, as follows:
[0044] CaCO 3 · MgCO 3 =CaO+MgO+2CO 2
[0045] CaCO 3 =CaO+CO 2
[0046] At the same time, fluoroapatite (Ca 5 F(PO 4 ) 3 ) has a decomposition temperature of 1650°C and will not decompose in the range of 900-1100°C;
[0047] (2) Digest the calcined slag with water at 60°C; using higher temperature water can accelerate...
Embodiment 2
[0070] A process for preparing phosphate concentrate by-products calcium carbonate and magnesium oxide from medium and low-grade phosphate rock, the process flow chart is as follows figure 1 shown, including the following steps:
[0071] (1) Calcining 100g of low- and medium-grade phosphate rock at 900-1100°C for 2 hours to obtain calcined slag and carbon dioxide; studies have shown that dolomite (CaCO 3 · MgCO 3 ), calcite (CaCO 3 ) and fluorapatite (Ca 5 F(PO 4 ) 3 ), where dolomite and calcite begin to decompose at high temperatures above 750°C, as follows:
[0072] CaCO 3 · MgCO 3 =CaO+MgO+2CO 2
[0073] CaCO 3 =CaO+CO 2
[0074] At the same time, fluoroapatite (Ca 5 F(PO 4 ) 3 ) has a decomposition temperature of 1650°C and will not decompose in the range of 900-1100°C;
[0075] (2) Digest the calcined slag with water at 100°C; using higher temperature water can accelerate the reaction of CaO and MgO with water to form calcium hydroxide and magnesium hydro...
Embodiment 3
[0089] A process for preparing phosphate concentrate by-products calcium carbonate and magnesium oxide from medium and low-grade phosphate rock, the process flow chart is as follows figure 1 shown, including the following steps:
[0090](1) Calcining 100g of low- and medium-grade phosphate rock at 900-1100°C for 1.5 hours to obtain calcined slag and carbon dioxide; studies have shown that dolomite (CaCO 3 · MgCO 3 ), calcite (CaCO 3 ) and fluorapatite (Ca 5 F(PO 4 ) 3 ), where dolomite and calcite begin to decompose at high temperatures above 750°C, as follows:
[0091] CaCO 3 · MgCO 3 =CaO+MgO+2CO 2
[0092] CaCO 3 =CaO+CO 2
[0093] At the same time, fluoroapatite (Ca 5 F(PO 4 ) 3 ) has a decomposition temperature of 1650°C and will not decompose in the range of 900-1100°C;
[0094] (2) Digest the calcined slag with water at 80°C; the use of higher temperature water can accelerate the reaction of CaO and MgO with water to form calcium hydroxide and magnesium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com